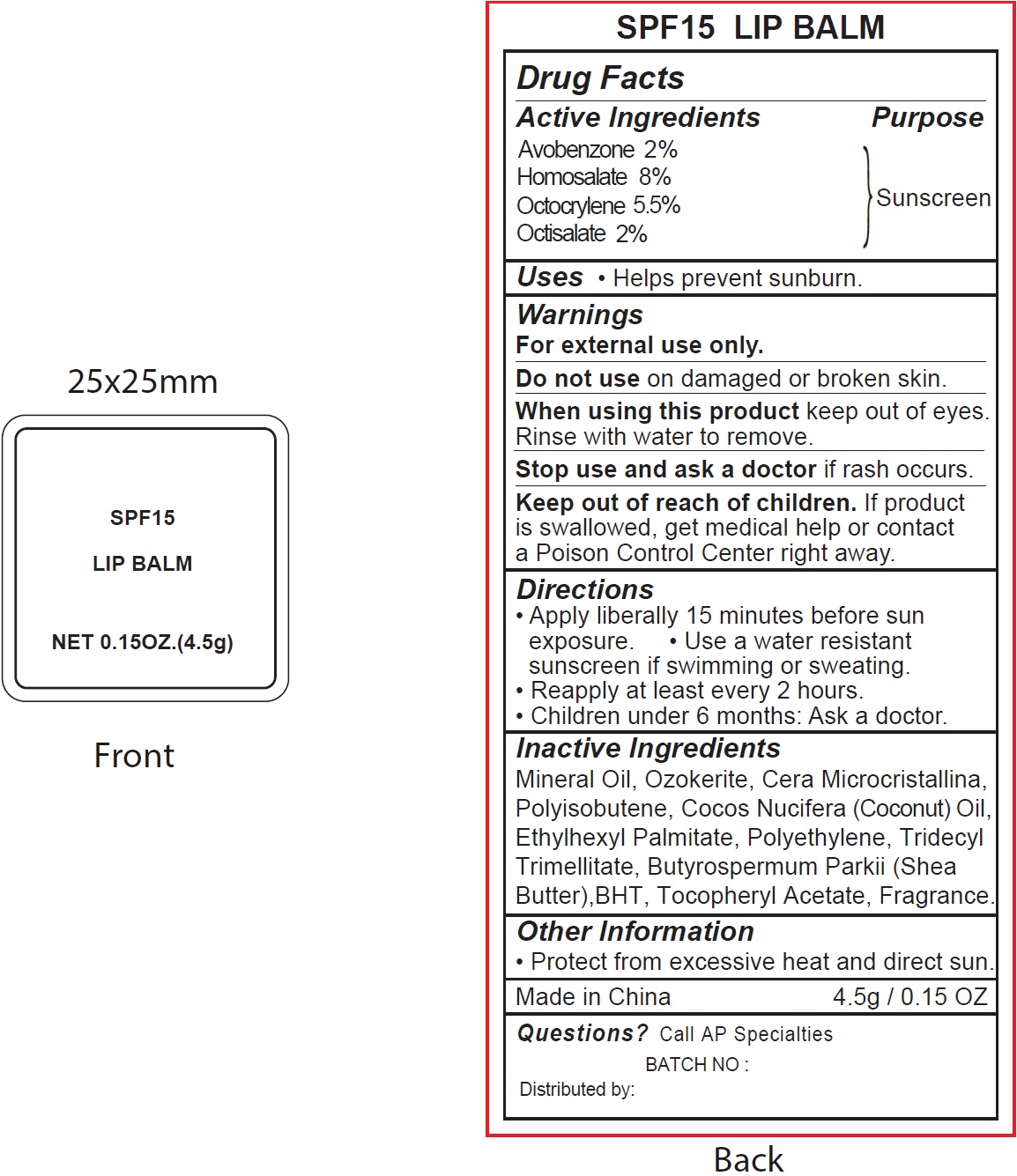
SPF15- avobenzone, homosalate, octocrylene, octisalate lipstick
Zhejiang Ayan Biotech Co.,Ltd.
----------
Active Ingredients
Avobenzone 2% Octocrylene 8% Octisalate 5.5% Homosalate 2%
Warnings
For external use only.
Do not use
on damaged or broken skin.
When using this product
keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor
if reash occurs.
Keep out of reach of children.
If product is swallowed, get medical help or contact a Poison Control right away.
Directions
- Apply liberally 15 minutes before sun exposure.
- Usa a water resistant sunscreen if swimming or sweating.
- Reapply at least every 2 hours.
- Children under 6 months: Ask a doctor.
Inactive Ingredients
Mineral Oil, Ozokerite, Cera Microcristallina, Polyisobutene, Cocos Nucifera (Coconut) Oil, Ethylhexyl Palmitate, Polyethylene, Tridencyl Trimellitate, Butyrospermum Parkii (Shea Butter), BHT, Tocopheryl Acetate, Fragrance.
Other Information
- Protect from excessive heat and direct sun.
Questions?
Call AP Specialties
BATCH NO:
Distributed by:
Package Labeling:
Zhejiang Ayan Biotech Co.,Ltd.