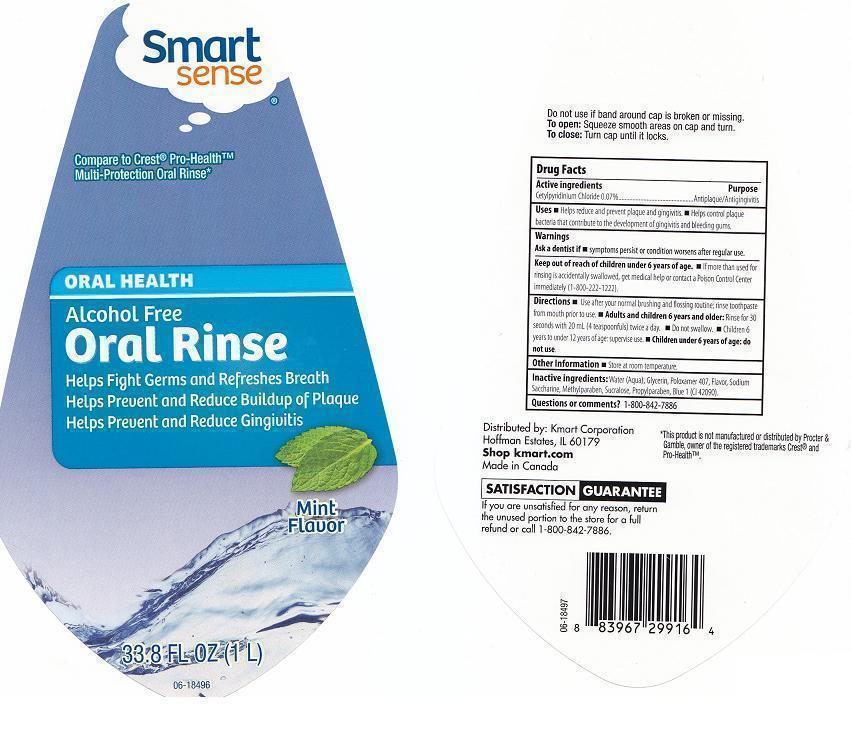
SMART SENSE ALCOHOL FREE- cetylpyridinium chloride liquid
KMART CORPORATION
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
ACTIVE INGREDIENTS
CETYLPYRIDINIUM CHLORIDE 0.07%
PURPOSE
ANTIPLAQUE/ANTIGINGIVITIS
USES
HELPS REDUCE AND PREVENT PLAQUE AND GINGIVITIS. HELPS CONTROL PLAQUE BACTERIA THAT CONTRIBUTE TO THE DEVELOPMENT OF GINGIVITIS AND BLEEDING GUMS.
WARNINGS
ASK A DENTIST IF SYMPTOMS PERSIST OR CONDITION WORSENS AFTER REGULAR USE.
KEEP OUT OF REACH OF CHILDREN UNDER 6 YEARS OF AGE.
IF MORE THAN USED FOR RINSING IS ACCIDENTALLY SWALLOWED, GET MEDICAL HELP OR CONTACT A POISON CONTROL CENTER IMMEDIATELY (1-800-222-1222).
DIRECTIONS
USE AFTER YOUR NORMAL BRUSHING AND FLOSSING ROUTINE; RINSE TOOTHPASTE FROM MOUTH PRIOR TO USE. ADULTA AND CHILDREN 6 YEARS AND OLDER: RINSE FOR 30 SECONDS WITH 20 ML (4 TEASPOONFULS) TWICE A DAY. DO NOT SWALLOW. CHILDREN 6 YEARS TO UNDER 12 YEARS OF AGE: SUPERVISE USE. CHILDREN UNDER 6 YEARS OF AGE: DO NOT USE.
OTHER INFORMATION
STORE AT ROOM TEMPERATURE.
INACTIVE INGREDIENTS:
WATER (AQUA), GLYCERIN, POLOXAMER 407, FLAVOR, SODIUM SACCHARIN, METHYLPARABEN, SUCRALOSE, PROPYLPARABEN, BLUE 1 (CI 42090).
QUESTIONS OR COMMENTS?
1-800-842-7886
LABEL COPY