Uses:
- Temporarily relieves these symptoms associated with the common cold, hay fever, or other respiratory allergies.
- Sneezing.
- Nasal congestion.
- Runny nose.
- Itchy, watery eyes.
Ask a doctor or pharmacist before use
If you have- Trouble urinating due to enlarged prostate gland
- A breathing problem such as emphysema or chronic bronchitis
- Glaucoma
- If you are taking sedatives or tranquilizers
When using this product
- Avoid alcoholic drinks.
- Marked drowsiness may occur.
- Excitability may occur, especially in children.
- Alcohol, sedatives and tranquilizers may increase drowsiness.
- Be careful when driving a motor vehicle or operating machinery.
Keep out of reach of children
In case of overdose, get medical help or contact a Poison Control Center right away.Directions:
- Take every 4-6 hours
- Do not take more than 6 doses in 24 hours.
| Adults and children 12 years or over | 1 to 2 capsule |
| Children 6 to under 12 years | 1 capsule |
| Children under 6 years | ask a doctor |
Other information:
- Store at room temperature 15-30 degrees C (59-86 degrees F)
- Protect from excessive moisture
Inactive ingredients:
Black Iron Oxide, D & C Red #28, FD & C Blue #1, FD & C Red #40, Gelatin, Lactose Monohydrate, Magnesium Stearate, Silicon Dioxide, Sodium Lauryl Sulfate
HOW SUPPLIED
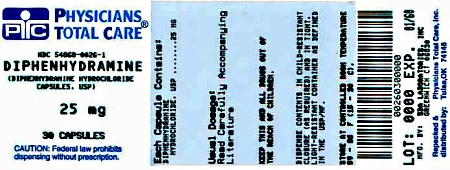
Diphenhydramine HCL 25 mg Capsules
| Bottles of 10 | NDC 54868-0026-5 |
| Bottles of 20 | NDC 54868-0026-6 |
| Bottles of 30 | NDC 54868-0026-1 |
| Bottles of 60 | NDC 54868-0026-7 |
| Bottles of 90 | NDC 54868-0026-8 |
| Bottles of 100 | NDC 54868-0026-0 |
| Bottles of 1000 | NDC 54868-0026-4 |
Relabeling and Repackaging by:
Physicians Total Care, Inc.
Tulsa, Oklahoma 74146