INDICATIONS AND USAGE
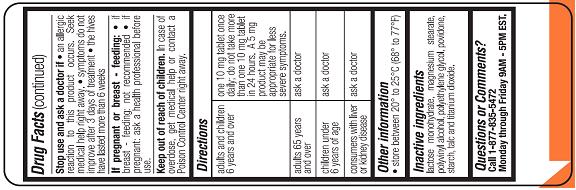
Relieves itching due to hives (urticaria). This product will not prevent hives or an allergic skin reaction from occurring.
WARNINGS
Severe Allergy Warning: Get emergency help immediately if you have hives along
with any of the following symptoms:
• trouble swallowing
• dizziness or loss of consciousness
• swelling of tongue
• swelling in or around mouth
• trouble speaking
• drooling
• wheezing or problems breathing
These symptoms may be signs of anaphylactic shock. This condition can be life threatening if not treated by a health professional immediately. Symptoms of anaphylactic shock may occur when hives first appear or up to a few hours later.
Not a Substitute for Epinephrine . If your doctor has prescribed an epinephrine injector for “anaphylaxis” or severe allergy symptoms that could occur with your hives, never use this product as a substitute for the epinephrine injector. If you have been prescribed an epinephrine injector, you should carry it with you at all times.
DO NOT USE
Do not use
• to prevent hives from any known cause such as:
• foods • insect stings • medicines • latex or rubber gloves
because this product will not stop hives from occurring. Avoiding the cause of your hives is the only way to prevent them. Hives can sometimes be serious. If you do not know the cause of your hives, see your doctor for medical exam. Your doctor may be able to help you find a cause.
• if you have ever had an allergic reaction to this product or its ingredients or to an antihistamine containing hydroxyzine.
ASK DOCTOR
Ask a doctor before use if you have
• liver or kidney disease. Your doctor should determine if you need a different dose.
• hives that are an unusual color, look bruised or blistered
• hives that do not itch
ASK DOCTOR/PHARMACIST
Ask a doctor of pharmacist before use if you are taking tranquilizers or sedatives.
STOP USE
Stop use and ask a doctor if
• an allergic reaction to this product occurs. Seek medical help right away.
• symptoms do not improve after 3 days of treatment
• the hives have lasted more than 6 weeks
WHEN USING
When using this product
• drowsiness may occur
• avoid alcoholic drinks
• alcohol, sedatives, and tranquilizers may increase drowsiness
• be careful when driving a motor vehicle or operating machinery
PREGNANCY OR BREAST FEEDING
If pregnant or breast-feeding:
• if breast-feeding: not recommended
• if pregnant: ask a health professional before use.
KEEP OUT OF REACH OF CHILDREN
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
DOSAGE AND ADMINISTRATION
| Adults andchildren 6years andover | One 10 mg tablet once daily; do not take more than one 10 mg tablet in 24 hours. A 5 mg product may be appropriate for less sever symptoms |
| Adults 65years andover | Ask a doctor |
| Childrenunder 6 yearsof age | ask a doctor |
| Consumerswith liver orkidney disease | ask a doctor |