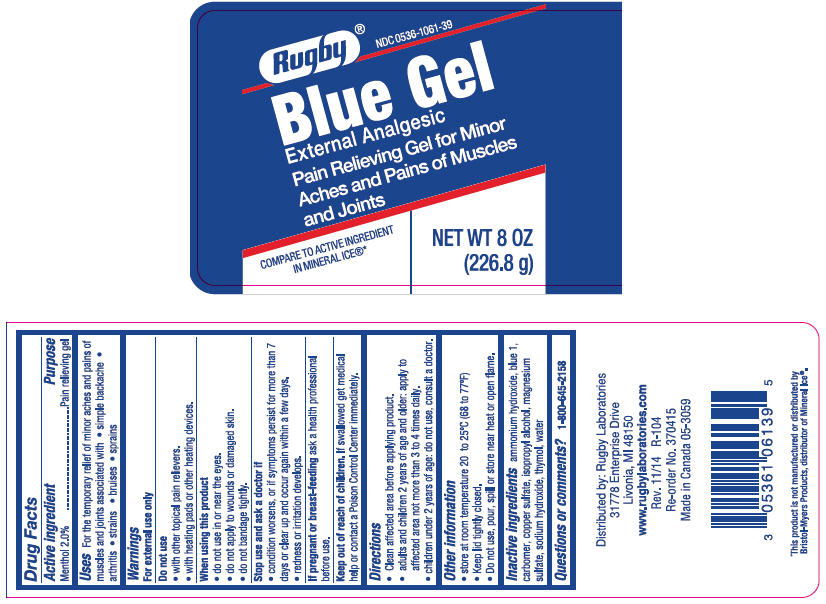
Active ingredient
Menthol 2.0%
Purpose
Pain relieving gel
Uses
For the temporary relief of minor aches and pains of muscles and joints associated with
- simple backache
- arthritis
- strains
- bruises
- sprains
Warnings
For external use only
Do not use
- with other topical pain relievers.
- with heating pads or other heating devices.
When using this product
- do not use in or near the eyes.
- do not apply to wounds or damaged skin.
- do not bandage tightly.
Stop use and ask a doctor if
- condition worsens, or if symptoms persist for more than 7 days or clear up and occur again within a few days.
- redness or irritation develops.
If pregnant or breast-feeding ask a health professional before use.
Keep out of reach of children. If swallowed get medical help or contact a Poison Control Center immediately.
Directions
- Clean affected area before applying product.
- adults and children 2 years of age and older: apply to affected area not more than 3 to 4 times daily.
- children under 2 years of age: do not use, consult a doctor.
Other information
- store at room temperature 20 to 25°C (68 to 77°F)
- Keep lid tightly closed.
- Do not use, pour, spill or store near heat or open flame.
Inactive ingredients
ammonium hydroxide, blue 1, carbomer, copper sulfate, isopropyl alcohol, magnesium sulfate, sodium hydroxide, thymol, water
Questions or comments?
1-800-645-2158
Distributed by: Rugby Laboratories
31778 Enterprise Drive
Livonia, MI 48150
PRINCIPAL DISPLAY PANEL - 226.8 g Jar Label
Rugby® NDC 0536-1061-39
Blue Gel
External Analgesic
Pain Relieving Gel for Minor
Aches and Pains of Muscles
and Joints
COMPARE TO ACTIVE INGREDIENT
IN MINERAL ICE®*
NET WT 8 OZ
(226.8 g)
Rugby Laboratories, Inc.