FULL PRESCRIBING INFORMATION
WARNING: EMBRYO-FETAL TOXICITY and VENOUS AND ARTERIAL THROMBOEMBOLISM
Embryo-Fetal Toxicity
- •
- POMALYST is contraindicated in pregnancy. POMALYST is a thalidomide analogue. Thalidomide is a known human teratogen that causes severe birth defects or embryo-fetal death. In females of reproductive potential, obtain 2 negative pregnancy tests before starting POMALYST treatment.
- •
- Females of reproductive potential must use 2 forms of contraception or continuously abstain from heterosexual sex during and for 4 weeks after stopping POMALYST treatment [see Contraindications (4), Warnings and Precautions (5.1) and Use in Specific Populations (8.1, 8.3)].
POMALYST is only available through a restricted distribution program called the POMALYST REMS [see Warnings and Precautions (5.2)]. Information about the POMALYST REMS program is available at www.pomalystrems.com or by calling the REMS Call Center at 1-888-423-5436.
Venous and Arterial Thromboembolism
- •
- Deep venous thrombosis (DVT), pulmonary embolism (PE), myocardial infarction, and stroke occur in patients with multiple myeloma treated with POMALYST. Prophylactic antithrombotic measures were employed in clinical trials. Thromboprophylaxis is recommended, and the choice of regimen should be based on assessment of the patient's underlying risk factors [see Warnings and Precautions (5.3)].
1 INDICATIONS AND USAGE
1.1 Multiple Myeloma
POMALYST, in combination with dexamethasone, is indicated for adult patients with multiple myeloma (MM) who have received at least two prior therapies including lenalidomide and a proteasome inhibitor and have demonstrated disease progression on or within 60 days of completion of the last therapy.
1.2 Kaposi Sarcoma
POMALYST is indicated for the treatment of:
- •
- Adult patients with AIDS-related Kaposi sarcoma (KS) after failure of highly active antiretroviral therapy (HAART).
- •
- Kaposi sarcoma (KS) in adult patients who are HIV-negative.
This indication is approved under accelerated approval based on overall response rate [see Clinical Studies (14.2)]. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial(s).
2 DOSAGE AND ADMINISTRATION
2.1 Pregnancy Testing Prior to Administration
Females of reproductive potential must have negative pregnancy testing and use contraception methods before initiating POMALYST [see Warnings and Precautions (5.1) and Use in Specific Populations (8.1, 8.3)].
2.2 Recommended Dosage for Multiple Myeloma
The recommended dosage of POMALYST is 4 mg once daily orally with or without food on Days 1 through 21 of each 28-day cycle until disease progression. Give POMALYST in combination with dexamethasone [see Clinical Studies (14.1)].
2.3 Recommended Dosage for Kaposi Sarcoma
The recommended dosage of POMALYST is 5 mg once daily taken orally with or without food on Days 1 through 21 of each 28-day cycle until disease progression or unacceptable toxicity. Continue HAART as HIV treatment in patients with AIDS-related Kaposi sarcoma (KS) [see Clinical Studies (14.2)].
2.4 Dosage Modifications for Hematologic Adverse Reactions
Multiple Myeloma: Dosage Modifications for Hematologic Adverse Reactions
Initiate a new cycle of POMALYST in patients with multiple myeloma (MM) when the neutrophil count is at least 500 per mcL and the platelet count is at least 50,000 per mcL.
Dosage modification for POMALYST for hematologic adverse reactions in patients with MM are summarized in Table 1.
| Adverse Reaction | Severity | Dosage Modification |
|---|---|---|
| * Permanently discontinue POMALYST if unable to tolerate 1 mg once daily. ANC= absolute neutrophil count |
||
|
Neutropenia [see Warnings and Precautions (5.5)] |
|
|
|
|
|
|
Thrombocytopenia [see Warnings and Precautions (5.5)] |
|
|
|
|
|
Kaposi Sarcoma: Dosage Modifications for Hematologic Adverse Reactions
Initiate a new cycle of POMALYST in patients with KS when the neutrophil count is at least 1000 per mcL and the platelet count is at least 75,000 per mcL.
Dose modifications for POMALYST for hematologic adverse reactions in patients with KS are summarized in Table 2.
| Adverse Reaction | Severity | Dosage Modification |
|---|---|---|
| * Permanently discontinue POMALYST if unable to tolerate 1mg once daily. ANC= absolute neutrophil count |
||
|
Neutropenia [see Warnings and Precautions (5.5)] |
ANC 500 to less than 1,000 per mcL |
Day 1 of cycle
During cycle
|
|
ANC less than 500 per mcL |
|
|
|
Febrile Neutropenia [see Warnings and Precautions (5.5)] |
ANC less than 1,000 per mcL and single temperature greater than or equal to 38.3°C |
|
|
Thrombocytopenia [see Warnings and Precautions (5.5)] |
Platelet count 25,000 to less than 50,000 per mcL |
Day 1 of cycle
During cycle:
|
|
Platelet count less than 25,000 per mcL |
Permanently discontinue POMALYST. |
|
2.5 Dosage Modifications for Non-Hematologic Adverse Reactions
Permanently discontinue POMALYST for angioedema, anaphylaxis, Grade 4 rash, skin exfoliation, bullae, or any other severe dermatologic reaction [See Warnings and Precautions (5.7, 5.12)].
For other Grade 3 or 4 toxicities, hold treatment and restart treatment at 1 mg less than the previous dose when toxicity has resolved to less than or equal to Grade 2 at the physician's discretion.
2.6 Dosage Modifications for Strong CYP1A2 Inhibitors
Avoid concomitant use of POMALYST with strong CYP1A2 inhibitors. If concomitant use of a strong CYP1A2 inhibitor is unavoidable, reduce POMALYST dose to 2 mg [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)].
2.7 Dosage Modification for Severe Renal Impairment on Hemodialysis
Take POMALYST after completion of dialysis procedure on hemodialysis days [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)].
- •
- For patients with MM with severe renal impairment requiring dialysis, reduce the recommended dosage to 3 mg orally daily.
- •
- For patients with KS with severe renal impairment requiring dialysis, reduce the recommended dosage to 4 mg orally daily.
2.8 Dosage Modification for Hepatic Impairment
Multiple Myeloma
For patients with MM with mild or moderate hepatic impairment (Child-Pugh A or B), reduce the recommended dosage to 3 mg orally daily.
For patients with MM with severe hepatic impairment (Child-Pugh C), reduce the recommended dosage to 2 mg [see Use in Specific Populations (8.7) and Clinical Pharmacology (12.3)].
Kaposi Sarcoma
For patients with KS with mild, moderate, or severe hepatic impairment (Child-Pugh A, B, or C), reduce the recommended dosage to 3 mg orally daily [see Use in Specific Populations (8.7) and Clinical Pharmacology (12.3)].
3 DOSAGE FORMS AND STRENGTHS
- •
- Capsules:1 mg, dark blue opaque cap and yellow opaque body, imprinted "POML" on the cap in white ink and "1 mg" on the body in black ink
- •
- 2 mg, dark blue opaque cap and orange opaque body, imprinted "POML" on the cap and "2 mg" on the body in white ink
- •
- 3 mg, dark blue opaque cap and green opaque body, imprinted "POML" on the cap and "3 mg" on the body in white ink
- •
- 4 mg, dark blue opaque cap and blue opaque body, imprinted "POML" on the cap and "4 mg" on the body in white ink
4 CONTRAINDICATIONS
4.1 Pregnancy
POMALYST is contraindicated in females who are pregnant. POMALYST can cause fetal harm when administered to a pregnant female [see Warnings and Precautions (5.1) and Use in Specific Populations (8.1)]. Pomalidomide is a thalidomide analogue and is teratogenic in both rats and rabbits when administered during the period of organogenesis. If the patient becomes pregnant while taking this drug, the patient should be apprised of the potential risk to a fetus.
4.2 Hypersensitivity
POMALYST is contraindicated in patients who have demonstrated severe hypersensitivity (e.g., angioedema, anaphylaxis) to pomalidomide or any of the excipients [see Warnings and Precautions (5.7), Description (11)].
5 WARNINGS AND PRECAUTIONS
5.1 Embryo-Fetal Toxicity
POMALYST is a thalidomide analogue and is contraindicated for use during pregnancy. Thalidomide is a known human teratogen that causes severe birth defects or embryo-fetal death [see Use in Specific Populations (8.1)]. POMALYST is only available through the POMALYST REMS program [see Warnings and Precautions (5.2)].
Females of Reproductive Potential
Females of reproductive potential must avoid pregnancy for at least 4 weeks before beginning POMALYST therapy, during therapy, during dose interruptions and for at least 4 weeks after completing therapy.
Females must commit either to abstain continuously from heterosexual sexual intercourse or to use 2 methods of reliable birth control, beginning 4 weeks prior to initiating treatment with POMALYST, during therapy, during dose interruptions, and continuing for 4 weeks following discontinuation of POMALYST therapy.
Two negative pregnancy tests must be obtained prior to initiating therapy. The first test should be performed within 10-14 days and the second test within 24 hours prior to prescribing POMALYST therapy and then weekly during the first month, then monthly thereafter in females with regular menstrual cycles, or every 2 weeks in females with irregular menstrual cycles [see Use in Specific Populations (8.3)].
Males
Pomalidomide is present in the semen of patients receiving the drug. Therefore, males must always use a latex or synthetic condom during any sexual contact with females of reproductive potential while taking POMALYST and for up to 4 weeks after discontinuing POMALYST, even if they have undergone a successful vasectomy. Male patients taking POMALYST must not donate sperm [see Use in Specific Populations (8.3)].
5.2 POMALYST REMS Program
Because of the embryo-fetal risk [see Warnings and Precautions (5.1)], POMALYST is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS), the "POMALYST REMS" program.
Required components of the POMALYST REMS program include the following:
- •
- Prescribers must be certified with the POMALYST REMS program by enrolling and complying with the REMS requirements.
- •
- Patients must sign a Patient-Physician Agreement Form and comply with the REMS requirements. In particular, female patients of reproductive potential who are not pregnant must comply with the pregnancy testing and contraception requirements [see Use in Specific Populations (8.3)] and males must comply with contraception requirements [see Use in Specific Populations (8.3)].
- •
- Pharmacies must be certified with the POMALYST REMS program, must only dispense to patients who are authorized to receive POMALYST and comply with REMS requirements.
Further information about the POMALYST REMS program is available at www.pomalystrems.com or by telephone at 1-888-423-5436.
5.3 Venous and Arterial Thromboembolism
Venous thromboembolic events (deep venous thrombosis and pulmonary embolism) and arterial thromboembolic events (myocardial infarction and stroke) have been observed in patients treated with POMALYST. In Trial 2, where anticoagulant therapies were mandated, thromboembolic events occurred in 8.0% of patients treated with POMALYST and low dose-dexamethasone (Low-dose Dex), and 3.3% of patients treated with high-dose dexamethasone. Venous thromboembolic events (VTE) occurred in 4.7% of patients treated with POMALYST and Low-dose Dex, and 1.3% of patients treated with high-dose dexamethasone. Arterial thromboembolic events include terms for arterial thromboembolic events, ischemic cerebrovascular conditions, and ischemic heart disease. Arterial thromboembolic events occurred in 3.0% of patients treated with POMALYST and Low-dose Dex, and 1.3% of patients treated with high-dose dexamethasone.
Patients with known risk factors, including prior thrombosis, may be at greater risk, and actions should be taken to try to minimize all modifiable factors (e.g., hyperlipidemia, hypertension, smoking). Thromboprophylaxis is recommended, and the choice of regimen should be based on assessment of the patient's underlying risk factors.
5.4 Increased Mortality in Patients with Multiple Myeloma When Pembrolizumab Is Added to a Thalidomide Analogue and Dexamethasone
In two randomized clinical trials in patients with MM, the addition of pembrolizumab to a thalidomide analogue plus dexamethasone, a use for which no PD-1 or PD-L1 blocking antibody is indicated, resulted in increased mortality. Treatment of patients with MM with a PD-1 or PD-L1 blocking antibody in combination with a thalidomide analogue plus dexamethasone is not recommended outside of controlled clinical trials.
5.5 Hematologic Toxicity
Multiple Myeloma
In trials 1 and 2 in patients who received POMALYST + Low-dose Dex, neutropenia was the most frequently reported Grade 3 or 4 adverse reaction, followed by anemia and thrombocytopenia. Neutropenia of any grade was reported in 51% of patients in both trials. The rate of Grade 3 or 4 neutropenia was 46%. The rate of febrile neutropenia was 8%.
Monitor patients for hematologic toxicities, especially neutropenia. Monitor complete blood counts weekly for the first 8 weeks and monthly thereafter. Patients may require dose interruption and/or modification [see Dosage and Administration (2.4)].
Kaposi Sarcoma
In Trial 12-C-0047, hematologic toxicities were the most common (all grades and Grade 3 or 4) adverse reactions [see Adverse Reactions (6.1)]. Fifty percent of patients had Grade 3 or 4 neutropenia. Monitor patients for hematologic toxicities, especially decreased neutrophils. Monitor complete blood counts every 2 weeks for the first 12 weeks and monthly thereafter. Withhold, reduce the dose, or permanently discontinue POMALYST based on the severity of the reaction [see Dosage and Administration (2.4)].
5.6 Hepatotoxicity
Hepatic failure, including fatal cases, has occurred in patients treated with POMALYST. Elevated levels of alanine aminotransferase and bilirubin have also been observed in patients treated with POMALYST. Monitor liver function tests monthly. Stop POMALYST upon elevation of liver enzymes and evaluate. After return to baseline values, treatment at a lower dose may be considered.
5.7 Severe Cutaneous Reactions
Severe cutaneous reactions including Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and drug reaction with eosinophilia and systemic symptoms (DRESS) have been reported. DRESS may present with a cutaneous reaction (such as rash or exfoliative dermatitis), eosinophilia, fever, and/or lymphadenopathy with systemic complications such as hepatitis, nephritis, pneumonitis, myocarditis, and/or pericarditis. These reactions can be fatal. Consider POMALYST interruption or discontinuation for Grade 2 or 3 skin rash. Permanently discontinue POMALYST for Grade 4 rash, exfoliative or bullous rash, or for other severe cutaneous reactions such as SJS, TEN or DRESS [see Dosage and Administration (2.5)].
5.8 Dizziness and Confusional State
In trials 1 and 2 in patients who received POMALYST + Low-dose Dex, 14% of patients experienced dizziness and 7% of patients experienced a confusional state; 1% of patients experienced Grade 3 or 4 dizziness, and 3% of patients experienced Grade 3 or 4 confusional state. Instruct patients to avoid situations where dizziness or confusional state may be a problem and not to take other medications that may cause dizziness or confusional state without adequate medical advice.
5.9 Neuropathy
In trials 1 and 2 in patients who received POMALYST + Low-dose Dex, 18% of patients experienced neuropathy, with approximately 12% of the patients experiencing peripheral neuropathy. Two percent of patients experienced Grade 3 neuropathy in trial 2. There were no cases of Grade 4 neuropathy adverse reactions reported in either trial.
5.10 Risk of Second Primary Malignancies
Cases of acute myelogenous leukemia have been reported in patients receiving POMALYST as an investigational therapy outside of MM.
5.11 Tumor Lysis Syndrome
Tumor lysis syndrome (TLS) may occur in patients treated with POMALYST. Patients at risk for TLS are those with high tumor burden prior to treatment. These patients should be monitored closely and appropriate precautions taken.
5.12 Hypersensitivity
Hypersensitivity, including angioedema, anaphylaxis, and anaphylactic reactions to POMALYST have been reported. Permanently discontinue POMALYST for angioedema or anaphylaxis [see Dosage and Administration (2.5)].
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described in detail in other labeling sections:
- •
- Embryo-Fetal Toxicity [see Warnings and Precautions (5.1, 5.2)]
- •
- Venous and Arterial Thromboembolism [see Warnings and Precautions (5.3)]
- •
- Increased Mortality in Patients with Multiple Myeloma When Pembrolizumab Is Added to a Thalidomide Analogue and Dexamethasone [see Warnings and Precautions (5.4)]
- •
- Hematologic Toxicity [see Warnings and Precautions (5.5)]
- •
- Hepatotoxicity [see Warnings and Precautions (5.6)]
- •
- Severe Cutaneous Reactions [see Warnings and Precautions (5.7)]
- •
- Dizziness and Confusional State [see Warnings and Precautions (5.8)]
- •
- Neuropathy [see Warnings and Precautions (5.9)]
- •
- Risk of Second Primary Malignancies [see Warnings and Precautions (5.10)]
- •
- Tumor Lysis Syndrome [see Warnings and Precautions (5.11)]
- •
- Hypersensitivity [see Warnings and Precautions (5.12)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Multiple Myeloma (MM)
In Trial 1, data were evaluated from 219 patients (safety population) who received treatment with POMALYST + Low-dose Dex (112 patients) or POMALYST alone (107 patients). Median number of treatment cycles was 5. Sixty-seven percent of patients in the study had a dose interruption of either drug due to adverse reactions. Forty-two percent of patients in the study had a dose reduction of either drug due to adverse reactions. The discontinuation rate due to adverse reactions was 11%.
In Trial 2, data were evaluated from 450 patients (safety population) who received treatment with POMALYST + Low-dose Dex (300 patients) or High-dose Dexamethasone (High-dose Dex) (150 patients). The median number of treatment cycles for the POMALYST + Low-dose Dex arm was 5. In the POMALYST + Low-dose Dex arm, 67% of patients had a dose interruption of POMALYST, the median time to the first dose interruption of POMALYST was 4.1 weeks. Twenty-seven percent of patients had a dose reduction of POMALYST, the median time to the first dose reduction of POMALYST was 4.5 weeks. Eight percent of patients discontinued POMALYST due to adverse reactions.
Tables 3 and 4 summarize the adverse reactions reported in Trials 1 and 2, respectively.
| * Regardless of attribution of relatedness to POMALYST. a POMALYST alone arm includes all patients randomized to the POMALYST alone arm who took study drug; 61 of the 107 patients had dexamethasone added during the treatment period. b Serious adverse reactions were reported in at least 2 patients in any POMALYST treatment arm. Data cutoff: 01 March 2013 |
||||
|
All Adverse Reactions ≥10% in Either Arm |
Grade 3 or 4 ≥5% in Either Arm |
|||
|
Body System
|
POMALYSTa
|
POMALYST + Low-dose Dex
|
POMALYST
|
POMALYST + Low-dose Dex
|
|
Number (%) of patients with at least one adverse reaction |
107 (100) |
112 (100) |
98 (92) |
102 (91) |
|
Blood and lymphatic system disorders |
||||
|
Neutropenia b |
57 (53) |
55 (49) |
51 (48) |
46 (41) |
|
Anemia b |
41 (38) |
47 (42) |
25 (23) |
24 (21) |
|
Thrombocytopenia b |
28 (26) |
26 (23) |
24 (22) |
21 (19) |
|
Leukopenia |
14 (13) |
22 (20) |
7 (7) |
11 (10) |
|
Febrile neutropenia b |
<10% |
<10% |
6 (6) |
3 (3) |
|
Lymphopenia |
4 (4) |
17 (15) |
2 (2) |
8 (7) |
|
General disorders and administration site conditions |
||||
|
Fatigue and asthenia b |
62 (58) |
70 (63) |
13 (12) |
19 (17) |
|
Edema peripheral |
27 (25) |
19 (17) |
0 (0.0) |
0 (0.0) |
|
Pyrexia b |
25 (23) |
36 (32) |
<5% |
<5% |
|
Chills |
11 (10) |
14 (13) |
0 (0.0) |
0 (0.0) |
|
Gastrointestinal disorders |
||||
|
Nausea b |
39 (36) |
27 (24) |
<5% |
<5% |
|
Constipation b |
38 (36) |
41 (37) |
<5% |
<5% |
|
Diarrhea |
37 (35) |
40 (36) |
<5% |
<5% |
|
Vomiting b |
15 (14) |
16 (14) |
<5% |
0 (0.0) |
|
Musculoskeletal and connective tissue disorders |
||||
|
Back pain b |
37 (35) |
36 (32) |
15 (14) |
11 (10) |
|
Musculoskeletal chest pain |
25 (23) |
22 (20) |
<5% |
0 (0.0) |
|
Muscle spasms |
23 (21) |
22 (20) |
<5% |
<5% |
|
Arthralgia |
18 (17) |
17 (15) |
<5% |
<5% |
|
Muscular weakness |
15 (14) |
15 (13) |
6 (6) |
4 (4) |
|
Bone pain |
13 (12) |
8 (7) |
<5% |
<5% |
|
Musculoskeletal pain |
13 (12) |
19 (17) |
<5% |
<5% |
|
Pain in extremity |
8 (7) |
16 (14) |
0 (0.0) |
<5% |
|
Infections and infestations |
||||
|
Upper respiratory tract infection |
40 (37) |
32 (29) |
<5% |
<5% |
|
Pneumonia b |
30 (28) |
38 (34) |
21 (20) |
32 (29) |
|
Urinary tract infection b |
11 (10) |
19 (17) |
2 (2) |
10 (9) |
|
Sepsis b |
<10% |
<10% |
6 (6) |
5 (4) |
|
Metabolism and nutrition disorders |
||||
|
Decreased appetite |
25 (23) |
21 (19) |
<5% |
0 (0.0) |
|
Hypercalcemia b |
23 (21) |
13 (12) |
11 (10) |
1 (<1) |
|
Hypokalemia |
13 (12) |
13 (12) |
<5% |
<5% |
|
Hyperglycemia |
12 (11) |
17 (15) |
<5% |
<5% |
|
Hyponatremia |
12 (11) |
14 (13) |
<5% |
<5% |
|
Dehydration b |
<10% |
<10% |
5 (4.7) |
6 (5.4) |
|
Hypocalcemia |
6 (6) |
13 (12) |
0 (0.0) |
<5% |
|
Respiratory, thoracic and mediastinal disorders |
||||
|
Dyspnea b |
38 (36) |
50 (45) |
8 (7) |
14 (13) |
|
Cough |
18 (17) |
25 (22) |
0 (0.0) |
0 (0.0) |
|
Epistaxis |
18 (17) |
12 (11) |
<5% |
0 (0.0) |
|
Productive cough |
10 (9) |
14 (13) |
0 (0.0) |
0 (0.0) |
|
Oropharyngeal pain |
6 (6) |
12 (11) |
0 (0.0) |
0 (0.0) |
|
Nervous system disorders |
||||
|
Dizziness |
24 (22) |
20 (18) |
<5% |
<5% |
|
Peripheral neuropathy |
23 (21) |
20 (18) |
0 (0.0) |
0 (0.0) |
|
Headache |
16 (15) |
15 (13) |
0 (0.0) |
<5% |
|
Tremor |
11 (10) |
15 (13) |
0 (0.0) |
0 (0.0) |
|
Skin and subcutaneous tissue disorders |
||||
|
Rash |
22 (21) |
18 (16) |
0 (0.0) |
<5% |
|
Pruritus |
16 (15) |
10 (9) |
0 (0.0) |
0 (0.0) |
|
Dry skin |
10 (9) |
12 (11) |
0 (0.0) |
0 (0.0) |
|
Hyperhidrosis |
8 (7) |
18 (16) |
0 (0.0) |
0 (0.0) |
|
Night sweats |
5 (5) |
14 (13) |
0 (0.0) |
0 (0.0) |
|
Investigations |
||||
|
Blood creatinine increased b |
20 (19) |
11 (10) |
6 (6) |
3 (3) |
|
Weight decreased |
16 (15) |
10 (9) |
0 (0.0) |
0 (0.0) |
|
Weight increased |
1 (<1) |
12 (11) |
0 (0.0) |
0 (0.0) |
|
Psychiatric disorders |
||||
|
Anxiety |
14 (13) |
8 (7) |
0 (0.0) |
0 (0.0) |
|
Confusional state b |
13 (12) |
15 (13) |
6 (6) |
3 (3) |
|
Insomnia |
7 (7) |
18 (16) |
0 (0.0) |
0 (0.0) |
|
Renal and urinary disorders |
||||
|
Renal failure b |
16 (15) |
11 (10) |
9 (8) |
8 (7) |
| a Percentage did not meet the criteria to be considered as an adverse reaction for POMALYST for that category of event (i.e., all adverse events or Grade 3 or 4 adverse events). b Serious adverse reactions were reported in at least 3 patients in the POM + Low-dose Dex arm, AND at least 1% higher than the High-dose-Dex arm percentage. Data cutoff: 01 March 2013 |
||||
|
All Adverse Reactions
|
Grade 3 or 4
|
|||
|
Body System
|
POMALYST + Low-dose Dex
|
High-dose Dex
|
POMALYST + Low-dose Dex
|
High-dose Dex
|
|
Number (%) of patients with at least one adverse reaction |
297 (99) |
149 (99) |
259 (86) |
127 (85) |
|
Blood and lymphatic system disorders |
||||
|
Neutropenia b |
154 (51) |
31 (21) |
145 (48) |
24 (16) |
|
Thrombocytopenia |
89 (30) a |
44 (29) a |
66 (22) a |
39 (26) a |
|
Leukopenia |
38 (13) |
8 (5) |
27 (9) |
5 (3) |
|
Febrile neutropenia b |
28 (9) |
0 (0.0) |
28 (9) |
0 (0.0) |
|
General disorders and administration site conditions |
||||
|
Fatigue and asthenia |
140 (47) |
64 (43) |
26 (9) a |
18 (12) a |
|
Pyrexia b |
80 (27) |
35 (23) |
9 (3) a |
7 (5) a |
|
Edema peripheral |
52 (17) |
17 (11) |
4 (1) a |
3 (2) a |
|
Pain |
11 (4) a |
3 (2) a |
5 (2) |
1 (<1) |
|
Infections and infestations |
||||
|
Upper respiratory tract infection b |
93 (31) |
19 (13) |
9 (3) |
1 (<1) |
|
Pneumonia b |
58 (19) |
20 (13) |
47 (16) |
15 (10) |
|
Neutropenic sepsis b |
3 (1) a |
0 (0.0) a |
3 (1) |
0 (0.0) |
|
Gastrointestinal disorders |
||||
|
Diarrhea |
66 (22) |
28 (19) |
3 (1) a |
2 (1) a |
|
Constipation |
65 (22) |
22 (15) |
7 (2) |
0 (0.0) |
|
Nausea |
45 (15) |
17 (11) |
3 (1) a |
2 (1) a |
|
Vomiting |
23 (8) |
6 (4) |
3 (1) |
0 (0.0) |
|
Musculoskeletal and connective tissue disorders |
||||
|
Back pain b |
59 (20) |
24 (16) |
15 (5) |
6 (4) |
|
Bone pain b |
54 (18) |
21 (14) |
22 (7) |
7 (5) |
|
Muscle spasms |
46 (15) |
11 (7) |
1 (<1) a |
1 (<1) a |
|
Arthralgia |
26 (9) |
7 (5) |
2 (<1) a |
1 (<1) a |
|
Pain in extremity |
20 (7) a |
9 (6) a |
6 (2) |
0 (0.0) |
|
Respiratory, thoracic and mediastinal disorders |
||||
|
Dyspnea b |
76 (25) |
25 (17) |
17 (6) |
7 (5) |
|
Cough |
60 (20) |
15 (10) |
2 (<1) a |
1 (<1) a |
|
Chronic obstructive pulmonary disease b |
5 (2) a |
0 (0.0) a |
4 (1) |
0 (0.0) |
|
Nervous system disorders |
||||
|
Peripheral neuropathy |
52 (17) |
18 (12) |
5 (2) a |
2 (1) a |
|
Dizziness |
37 (12) |
14 (9) |
4 (1) a |
2 (1) a |
|
Headache |
23 (8) |
8 (5) |
1 (<1) a |
0 (0.0) a |
|
Tremor |
17 (6) |
2 (1) |
2 (<1) a |
0 (0.0) a |
|
Depressed level of consciousness |
5 (2) a |
0 (0.0) a |
3 (1) |
0 (0.0) |
|
Metabolism and nutrition disorders |
||||
|
Decreased appetite |
38 (13) |
12 (8) |
3 (1) a |
2 (1) a |
|
Hypokalemia |
28 (9) a |
12 (8) a |
12 (4) |
4 (3) |
|
Hypocalcemia |
12 (4) a |
9 (6) a |
5 (2) |
1 (<1) |
|
Skin and subcutaneous tissue disorders |
||||
|
Rash |
23 (8) |
2 (1) |
3 (1) |
0 (0.0) |
|
Pruritus |
22 (7) |
5 (3) |
0 (0.0) a |
0 (0.0) a |
|
Hyperhidrosis |
15 (5) |
1 (<1) |
0 (0.0) a |
0 (0.0) a |
|
Investigations |
||||
|
Neutrophil count decreased |
15 (5) |
1 (<1) |
14 (5) |
1 (<1) |
|
Platelet count decreased |
10 (3) a |
3 (2) a |
8 (3) |
2 (1) |
|
White blood cell count decreased |
8 (3) a |
1 (<1) a |
8 (3) |
0 (0.0) |
|
Alanine aminotransferase increased |
7 (2) a |
2 (1) a |
5 (2) |
0 (0.0) |
|
Aspartate aminotransferase increased |
4 (1) a |
2 (1) a |
3 (1) |
0 (0.0) |
|
Lymphocyte count decreased |
3 (1) a |
1 (<1) a |
3 (1) |
0 (0.0) |
|
Renal and urinary disorders |
||||
|
Renal failure |
31 (10) a |
18 (12) a |
19 (6) |
8 (5) |
|
Injury, poisoning and procedural complications |
||||
|
Femur fracture b |
5 (2) a |
1 (<1) a |
5 (2) |
1 (<1) |
|
Reproductive system and breast disorders |
||||
|
Pelvic pain |
6 (2) a |
3 (2) a |
4 (1) |
0 (0.0) |
Other Adverse Reactions
Other adverse reactions of POMALYST in patients with MM, not described above, and considered important:
Cardiac Disorders: Myocardial infarction, Atrial fibrillation, Angina pectoris, Cardiac failure congestive
Ear and Labyrinth Disorders: Vertigo
Gastrointestinal disorders: Abdominal pain
General Disorders and Administration Site Conditions: General physical health deterioration, Non-cardiac chest pain, Multi-organ failure
Hepatobiliary Disorders: Hyperbilirubinemia
Infections and Infestations: Pneumocystis jiroveci pneumonia, Respiratory syncytial virus infection, Neutropenic sepsis, Bacteremia, Pneumonia respiratory syncytial viral, Cellulitis, Urosepsis, Septic shock, Clostridium difficile colitis, Pneumonia streptococcal, Lobar pneumonia, Viral infection, Lung infection
Investigations: Alanine aminotransferase increased, Hemoglobin decreased
Injury, poisoning and procedural complications: Fall, Compression fracture, Spinal compression fracture
Metabolism and nutritional disorders: Hyperkalemia, Failure to thrive
Nervous system disorders: Depressed level of consciousness, Syncope
Psychiatric disorders: Mental status change
Renal and urinary disorders: Urinary retention, Hyponatremia
Reproductive system and breast disorders: Pelvic pain
Respiratory, thoracic, and mediastinal disorders: Interstitial lung disease, Pulmonary embolism, Respiratory failure, Bronchospasm
Vascular disorders: Hypotension
Kaposi Sarcoma (KS)
The safety of POMALYST in patients with KS was evaluated in Trial 12-C-0047 [see Clinical Studies (14.2)]. Twenty-eight patients received POMALYST 5 mg taken orally once daily on Days 1 through 21 of repeated 28-day cycles. The study excluded patients with procoagulant disorders or a history of venous or arterial thromboembolism. Patients received DVT prophylaxis with daily low dose aspirin. Across all patients treated on Trial 12-C-0047, 75% were exposed to pomalidomide for 6 months or longer and 25% were exposed for greater than one year.
Serious adverse reactions occurred in 18% (5/28) of patients who received POMALYST. The following serious adverse reactions each occurred in 1 patient: anemia, decreased neutrophil count, and hematuria.
Permanent discontinuation due to an adverse reaction occurred in 11% (3/28) of patients who received POMALYST.
Dosage interruptions due to an adverse reaction occurred in 14% (4/28) of patients who received POMALYST. The most frequent adverse reaction requiring dosage interruption was decreased neutrophil count, which occurred in 3 patients.
The POMALYST dose was reduced due to an adverse reaction in 1 patient due to gout.
Tables 5 and 6 summarize the adverse reactions and select laboratory abnormalities reported in Trial 12-C-0047.
|
Adverse Reaction |
Grades 1-4
|
Grade 3 or 4
|
|
Rash, maculo-papular |
71 |
3.6 |
|
Constipation |
71 |
0 |
|
Fatigue |
68 |
0 |
|
Nausea |
36 |
0 |
|
Diarrhea |
32 |
3.6 |
|
Cough |
29 |
0 |
|
Dyspnea |
29 |
0 |
|
Peripheral Edema |
29 |
3.6 |
|
Upper respiratory tract infection |
29 |
0 |
|
Muscle spasms |
25 |
0 |
|
Hypothyroidism |
21 |
0 |
|
Dry skin |
21 |
0 |
|
Chills |
21 |
0 |
| * Denominator is the number of patients for whom there is a baseline and at least one post baseline assessment for the laboratory parameter. | ||
|
Laboratory Abnormality |
Grades 1-4*
|
Grades 3-4*
|
|
Hematology |
||
|
Decreased Absolute Neutrophil Count |
96 |
50 |
|
Decreased White Blood Cells |
79 |
3.6 |
|
Decreased Hemoglobin |
54 |
0 |
|
Decreased Platelets |
54 |
0 |
|
Chemistry |
||
|
Elevated Creatinine |
86 |
3.6 |
|
Elevated Glucose |
57 |
7 |
|
Decreased Albumin |
54 |
0 |
|
Decreased Phosphate |
54 |
25 |
|
Decreased Calcium |
50 |
0 |
|
Increased Alanine Aminotransferase (ALT) |
32 |
0 |
|
Increased Aspartate Aminotransferase (AST) |
25 |
0 |
|
Elevated Creatine Kinase |
25 |
7 |
|
Decreased Magnesium |
14 |
0 |
|
Elevated Alkaline Phosphate |
14 |
3.6 |
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of POMALYST. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Blood and Lymphatic System Disorders: Pancytopenia
Endocrine Disorders: Hypothyroidism, hyperthyroidism
Gastrointestinal Disorders: Gastrointestinal hemorrhage
Hepatobiliary Disorders: Hepatic failure (including fatal cases), elevated liver enzymes
Immune system Disorders: Allergic reactions (e.g., angioedema, anaphylaxis, urticaria), solid organ transplant rejection
Infections and Infestations: Hepatitis B virus reactivation, Herpes zoster, progressive multifocal leukoencephalopathy (PML)
Neoplasms benign, malignant and unspecified (incl cysts and polyps): Tumor lysis syndrome, basal cell carcinoma, and squamous cell carcinoma of the skin
Skin and Subcutaneous Tissue Disorders: Stevens-Johnson Syndrome, toxic epidermal necrolysis, drug reaction with eosinophilia and systemic symptoms (DRESS)
7 DRUG INTERACTIONS
7.1 Drugs That Affect Pomalidomide Plasma Concentrations
CYP1A2 inhibitors:
In healthy subjects, co-administration of fluvoxamine, a strong CYP1A2 inhibitor, increased Cmax and AUC of pomalidomide by 24% and 125% respectively [see Clinical Pharmacology (12.3)]. Increased pomalidomide exposure may increase the risk of exposure related toxicities. Avoid co-administration of strong CYP1A2 inhibitors (e.g. ciprofloxacin and fluvoxamine). If co-administration is unavoidable, reduce the POMALYST dose [see Dosage and Administration (2.6)].
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in females exposed to POMALYST during pregnancy as well as female partners of male patients who are exposed to POMALYST. This registry is also used to understand the root cause for the pregnancy. Report any suspected fetal exposure to POMALYST to the FDA via the MedWatch program at 1-800-FDA-1088 and also to the REMS Call Center at 1-888-423-5436.
Risk Summary
Based on the mechanism of action [see Clinical Pharmacology (12.1)] and findings from animal studies, POMALYST can cause embryo-fetal harm when administered to a pregnant female and is contraindicated during pregnancy [see Contraindications (4), and Warnings and Precautions (5.1)].
POMALYST is a thalidomide analogue. Thalidomide is a human teratogen, inducing a high frequency of severe and life-threatening birth defects such as amelia (absence of limbs), phocomelia (short limbs), hypoplasticity of the bones, absence of bones, external ear abnormalities (including anotia, micropinna, small or absent external auditory canals), facial palsy, eye abnormalities (anophthalmos, microphthalmos), and congenital heart defects. Alimentary tract, urinary tract, and genital malformations have also been documented, and mortality at or shortly after birth has been reported in about 40% of infants.
Pomalidomide was teratogenic in both rats and rabbits when administered during the period of organogenesis. Pomalidomide crossed the placenta after administration to pregnant rabbits (see Data). If this drug is used during pregnancy or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential risk to a fetus.
If pregnancy does occur during treatment, immediately discontinue the drug. Under these conditions, refer patient to an obstetrician/gynecologist experienced in reproductive toxicity for further evaluation and counseling. Report any suspected fetal exposure to POMALYST to the FDA via the MedWatch program at 1-800-FDA-1088 and also to the REMS Call Center at 1-888-423-5436.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. The estimated background risk in the U.S. general population of major birth defects is 2%-4% and of miscarriage is 15%-20% of clinically recognized pregnancies.
Data
Animal Data
Pomalidomide was teratogenic in both rats and rabbits in the embryo-fetal developmental studies when administered during the period of organogenesis.
In rats, pomalidomide was administered orally to pregnant animals at doses of 25 to 1000 mg/kg/day. Malformations or absence of urinary bladder, absence of thyroid gland, and fusion and misalignment of lumbar and thoracic vertebral elements (vertebral, central, and/or neural arches) were observed at all dose levels. There was no maternal toxicity observed in this study. The lowest dose in rats resulted in an exposure (AUC) approximately 85-fold of the human exposure at the recommended dose of 4 mg/day. Other embryo-fetal toxicities included increased resorptions leading to decreased number of viable fetuses.
In rabbits, pomalidomide was administered orally to pregnant animals at doses of 10 to 250 mg/kg/day. Increased cardiac malformations such as interventricular septal defect were seen at all doses with significant increases at 250 mg/kg/day. Additional malformations observed at 250 mg/kg/day included anomalies in limbs (flexed and/or rotated fore- and/or hindlimbs, unattached or absent digit) and associated skeletal malformations (not ossified metacarpal, misaligned phalanx and metacarpal, absent digit, not ossified phalanx, and short not ossified or bent tibia), moderate dilation of the lateral ventricle in the brain, abnormal placement of the right subclavian artery, absent intermediate lobe in the lungs, low-set kidney, altered liver morphology, incompletely or not ossified pelvis, an increased average for supernumerary thoracic ribs, and a reduced average for ossified tarsals. No maternal toxicity was observed at the low dose (10 mg/kg/day) that resulted in cardiac anomalies in fetuses; this dose resulted in an exposure (AUC) approximately equal to that reported in humans at the recommended dose of 4 mg/day. Additional embryo-fetal toxicity included increased resorption.
Following daily oral administration of pomalidomide from Gestation Day 7 through Gestation Day 20 in pregnant rabbits, fetal plasma pomalidomide concentrations were approximately 50% of the maternal Cmax at all dosages (5 to 250 mg/kg/day), indicating that pomalidomide crossed the placenta.
8.2 Lactation
Risk Summary
There is no information regarding the presence of pomalidomide in human milk, the effects of POMALYST on the breastfed child, or the effects of POMALYST on milk production. Pomalidomide was excreted in the milk of lactating rats (see Data). Because many drugs are excreted in human milk and because of the potential for adverse reactions in a breastfed child from POMALYST, advise women not to breastfeed during treatment with POMALYST.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
POMALYST can cause fetal harm when administered during pregnancy [see Use in Specific Populations (8.1)]. Verify the pregnancy status of females of reproductive potential prior to initiating POMALYST therapy and during therapy. Advise females of reproductive potential that they must avoid pregnancy 4 weeks before therapy, while taking POMALYST, during dose interruptions and for at least 4 weeks after completing therapy.
Females of reproductive potential must have 2 negative pregnancy tests before initiating POMALYST. The first test should be performed within 10-14 days, and the second test within 24 hours prior to prescribing POMALYST. Once treatment has started and during dose interruptions, pregnancy testing for females of reproductive potential should occur weekly during the first 4 weeks of use, then pregnancy testing should be repeated every 4 weeks in females with regular menstrual cycles. If menstrual cycles are irregular, the pregnancy testing should occur every 2 weeks. Pregnancy testing and counseling should be performed if a patient misses her period or if there is any abnormality in her menstrual bleeding. POMALYST treatment must be discontinued during this evaluation.
Contraception
Females
Females of reproductive potential must commit either to abstain continuously from heterosexual sexual intercourse or to use 2 methods of reliable birth control simultaneously: one highly effective form of contraception – tubal ligation, IUD, hormonal (birth control pills, injections, hormonal patches, vaginal rings, or implants), or partner's vasectomy, and 1 additional effective contraceptive method – male latex or synthetic condom, diaphragm, or cervical cap. Contraception must begin 4 weeks prior to initiating treatment with POMALYST, during therapy, during dose interruptions, and continuing for 4 weeks following discontinuation of POMALYST therapy. Reliable contraception is indicated even where there has been a history of infertility, unless due to hysterectomy. Females of reproductive potential should be referred to a qualified provider of contraceptive methods, if needed.
Males
Pomalidomide is present in the semen of males who take POMALYST. Therefore, males must always use a latex or synthetic condom during any sexual contact with females of reproductive potential while taking POMALYST and for up to 4 weeks after discontinuing POMALYST, even if they have undergone a successful vasectomy. Male patients taking POMALYST must not donate sperm.
Infertility
Based on findings in animals, female fertility may be compromised by treatment with POMALYST [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
The safety and effectiveness of POMALYST have not been established in pediatric patients. The safety and effectiveness were assessed but not established in two open-label studies: a dose escalation study in 25 pediatric patients aged 5 to <17 with recurrent, progressive or refractory CNS tumors [NCT02415153] and a parallel-group study conducted in 47 pediatric patients aged 4 to <17 years with recurrent or progressive high-grade glioma, medulloblastoma, ependymoma, or diffuse intrinsic pontine glioma (DIPG) [NCT03257631]. No new safety signals were observed in pediatric patients across these studies.
At the same dose by body surface area, pomalidomide exposure in 55 pediatric patients aged 4 to < 17 years old was within the range observed in adult patients with MM but higher than the exposure observed in adult patients with KS [see Clinical Pharmacology (12.3)].
8.5 Geriatric Use
Multiple Myeloma
Of the total number of patients in clinical studies of POMALYST, 44% were aged older than 65 years, while 10% were aged older than 75 years. No overall differences in effectiveness were observed between these patients and younger patients. In these studies, patients older than 65 years were more likely than patients less than or equal to 65 years of age to experience pneumonia.
8.6 Renal Impairment
In patients with severe renal impairment requiring dialysis, the AUC of pomalidomide increased by 38% and the rate of SAE increased by 64% relative to patients with normal renal function; therefore, starting dose adjustment is recommended. For patients with severe renal impairment requiring dialysis, administer POMALYST after the completion of hemodialysis on dialysis days because exposure of pomalidomide could be significantly decreased during dialysis [see Dosage and Administration (2.7) and Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
Pomalidomide is metabolized primarily by the liver. Following single dose administration, the AUC of pomalidomide increased 51%, 58%, and 72% in subjects with mild (Child-Pugh class A), moderate (Child-Pugh class B), and severe (Child-Pugh class C) hepatic impairment, respectively compared to subjects with normal liver function. Dose adjustment is recommended in patients with hepatic impairment [see Dosage and Administration (2.8) and Clinical Pharmacology (12.3)].
8.8 Smoking Tobacco
Cigarette smoking reduces pomalidomide AUC due to CYP1A2 induction. Advise patients that smoking may reduce the efficacy of pomalidomide [see Clinical Pharmacology (12.3)].
11 DESCRIPTION
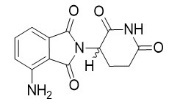
Pomalidomide is a thalidomide analog. The chemical name is (RS)-4-Amino-2-(2,6-dioxo-piperidin-3-yl)-isoindoline-1,3-dione and it has the following chemical structure:
The empirical formula for pomalidomide is C13H11N3O4 and the gram molecular weight is 273.24.
Pomalidomide is a yellow solid powder. It has limited to low solubility into organic solvents and it has low solubility in all pH solutions (about 0.01 mg/mL). Pomalidomide has a chiral carbon atom which exists as a racemic mixture of the R(+) and S(-) enantiomers.
POMALYST is available in 1-mg, 2-mg, 3-mg, and 4-mg capsules for oral administration. Each capsule contains pomalidomide as the active ingredient and the following inactive ingredients: mannitol, pregelatinized starch, and sodium stearyl fumarate. The 1-mg capsule shell contains gelatin, titanium dioxide, FD&C blue 2, yellow iron oxide, white ink, and black ink. The 2-mg capsule shell contains gelatin, titanium dioxide, FD&C blue 2, yellow iron oxide, FD&C red 3, and white ink. The 3-mg capsule shell contains gelatin, titanium dioxide, FD&C blue 2, yellow iron oxide, and white ink. The 4-mg capsule shell contains gelatin, titanium dioxide, FD&C blue 1, FD&C blue 2, and white ink.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Pomalidomide is an analogue of thalidomide with immunomodulatory, antiangiogenic, and antineoplastic properties. Cellular activities of pomalidomide are mediated through its target cereblon, a component of a cullin ring E3 ubiquitin ligase enzyme complex. In vitro, in the presence of drug, substrate proteins (including Aiolos and Ikaros) are targeted for ubiquitination and subsequent degradation leading to direct cytotoxic and immunomodulatory effects. In in vitro cellular assays, pomalidomide inhibited proliferation and induced apoptosis of hematopoietic tumor cells. Additionally, pomalidomide inhibited the proliferation of lenalidomide-resistant multiple myeloma (MM) cell lines and synergized with dexamethasone in both lenalidomide-sensitive and lenalidomide-resistant cell lines to induce tumor cell apoptosis. Pomalidomide enhanced T cell- and natural killer (NK) cell-mediated immunity and inhibited production of pro-inflammatory cytokines (e.g., TNF-α and IL-6) by monocytes. Pomalidomide demonstrated anti-angiogenic activity in a mouse tumor model and in the in vitro umbilical cord model.
12.2 Pharmacodynamics
Pomalidomide exposure-response analyses showed that there was no relationship between systemic pomalidomide exposure level and efficacy or safety following pomalidomide dose of 4 mg.
Cardiac Electrophysiology
The QTc prolongation potential of pomalidomide was evaluated in a single center, randomized, double-blind crossover study (N=72) using 4 mg pomalidomide, 20 mg pomalidomide, placebo, and 400 mg moxifloxacin (positive control). No significant QTc prolongation effect of pomalidomide was observed following pomalidomide doses of 4 and 20 mg.
12.3 Pharmacokinetics
In patients with MM who received POMALYST 4 mg daily alone or in combination with dexamethasone, pomalidomide steady-state drug exposure was characterized by AUC (CV%) of 860 (37%) ng∙h/mL and Cmax (CV%) of 75 (32%) ng/mL. In patients with Kaposi sarcoma (KS) who received POMALYST 5 mg daily, pomalidomide steady-state drug exposure was characterized by AUC of 462.3 ng∙h/mL (82%) and Cmax of 53.1 ng/mL (50%).
Absorption
Following administration of single oral doses of POMALYST, the maximum plasma concentration (Cmax) for pomalidomide occurs at 2 to 3 hours postdose in patients with MM or KS.
Effect of Food
Co-administration of POMALYST with a high-fat meal (approximately 50% of the total caloric content) and high-calorie meal (approximately 800 to 1000 calories) (the meal contained approximately 150, 250, and 500 to 600 calories from protein, carbohydrates, and fat, respectively) delays the Tmax by 2.5 hours, decreased mean plasma Cmax and AUC in healthy subjects by about 27% and 8%, respectively.
Distribution
Pomalidomide has a mean apparent volume of distribution (Vd/F) between 62 and 138 L at steady state in patients with MM or KS.
Pomalidomide is distributed in semen of healthy subjects at a concentration of approximately 67% of plasma level at 4 hours postdose (~Tmax) after 4 days of 2 mg once-daily dosing.
Human plasma protein binding of pomalidomide ranges from 12% to 44% and is not concentration dependent. Pomalidomide is a substrate for P-gp.
Elimination
Pomalidomide has a mean total body clearance (CL/F) of 7-10 L/h in patients with MM or KS. Pomalidomide is eliminated with a median plasma half-life of 9.5 hours in healthy subjects and 7.5 hours in patients with MM or KS.
Metabolism
Pomalidomide is primarily metabolized in the liver by CYP1A2 and CYP3A4. Minor contributions from CYP2C19 and CYP2D6 were also observed in vitro.
Excretion
Following a single oral administration of [14C]-pomalidomide to healthy subjects, approximately 73% and 15% of the radioactive dose was eliminated in urine and feces, respectively, with approximately 2% and 8% of the radiolabeled dose eliminated unchanged as pomalidomide in urine and feces.
Specific Populations
Age (61 to 85 years old), sex and race have no clinically significant effect on the systemic exposure of pomalidomide.
Patients with Renal Impairment
Pomalidomide pharmacokinetic parameters were not significantly affected in patients with moderate (30 mL/min ≤ CLcr< 60 mL/min) or severe (15 mL/min ≤ CLcr< 30 mL/min) renal impairment relative to patients with normal renal function (CLcr ≥ 60 mL/min). Mean exposure (AUC) to pomalidomide increased by 38% in patients with severe renal impairment requiring dialysis (CLcr< 30 mL/min requiring dialysis) and 40% in patients with end stage renal disease (CLcr< 15 mL/min) on non-dialysis days. In patients with severe renal impairment requiring dialysis, the estimated dialysis clearance is approximately 12 L/h which is higher than pomalidomide total body clearance, indicating hemodialysis will remove pomalidomide from the blood circulation.
Drug Interaction Studies
Clinical Studies
Co-administration of POMALYST with the following drugs did not increase pomalidomide exposure to a clinically significant extent: ketoconazole (a strong CYP3A4 and P-gp inhibitor), carbamazepine (a strong CYP3A4 inducer) and dexamethasone (a weak to moderate CYP3A4 inducer). Co-administration of POMALYST with drugs that are CYP1A2 inducers has not been studied.
CYP1A2 Inhibitors: Co-administration of fluvoxamine (a strong CYP1A2 inhibitor) with POMALYST increased mean [90% confidence interval] pomalidomide exposure by 125% [98% to 157%] compared to POMALYST alone in healthy subjects. Co-administration of fluvoxamine in the presence of ketoconazole (a strong CYP3A4 and P-gp inhibitor) with POMALYST increased mean pomalidomide exposure by 146% [126% to 167%] compared to POMALYST administered alone in healthy subjects, indicating the predominant effect of CYP1A2 inhibition in the increase of pomalidomide exposure [see Dosage and Administration (2.6) and Drug Interactions (7.1)].
Strong CYP3A4 and P-gp Inhibitors: Co-administration of ketoconazole (a strong CYP3A4 and P-gp inhibitor) in 16 healthy male subjects increased AUC of pomalidomide by 19% compared to POMALYST administered alone.
Strong CYP1A2 Inducers: Co-administration of POMALYST with drugs that are CYP1A2 inducers has not been studied and may reduce pomalidomide exposure.
Strong CYP3A4 Inducers: Co-administration of carbamazepine to 16 healthy male subjects decreased AUC of pomalidomide by 20% with a 90% confidence interval [13% to 27%] compared to when pomalidomide was administered alone.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies examining the carcinogenic potential of pomalidomide have not been conducted. One of 12 monkeys dosed with 1 mg/kg of pomalidomide (an exposure approximately 15-fold of the exposure in patients at the recommended dose of 4 mg/day) developed acute myeloid leukemia in a 9-month repeat-dose toxicology study.
Pomalidomide was not mutagenic or clastogenic in a battery of tests, including the bacteria reverse mutation assay (Ames test), the in vitro assay using human peripheral blood lymphocytes, and the micronucleus test in orally treated rats administered doses up to 2000 mg/kg/day.
In a fertility and early embryonic development study in rats, drug-treated males were mated with untreated or treated females. Pomalidomide was administered to males and females at doses of 25 to 1000 mg/kg/day. When treated males were mated with treated females, there was an increase in post-implantation loss and a decrease in mean number of viable embryos at all dose levels. There were no other effects on reproductive functions or the number of pregnancies. The lowest dose tested in animals resulted in an exposure (AUC) approximately 100-fold of the exposure in patients at the recommended dose of 4 mg/day. When treated males in this study were mated with untreated females, all uterine parameters were comparable to the controls. Based on these results, the observed effects were attributed to the treatment of females.
14 CLINICAL STUDIES
14.1 Multiple Myeloma
Trial 1
Trial 1 was a phase 2, multicenter, randomized open-label study in patients with relapsed multiple myeloma (MM) who were refractory to their last myeloma therapy and had received lenalidomide and bortezomib. Patients were considered relapsed if they had achieved at least stable disease for at least 1 cycle of treatment to at least 1 prior regimen and then developed progressive disease. Patients were considered refractory if they experienced disease progression on or within 60 days of their last therapy. A total of 221 patients were randomized to receive POMALYST alone or POMALYST with Low-dose Dex. In Trial 1, the safety and efficacy of POMALYST 4 mg, once daily for 21 of 28 days, until disease progression, were evaluated alone and in combination with Low-dose Dex (40 mg/day given only on Days 1, 8, 15, and 22 of each 28-day cycle for patients aged 75 years or younger, or 20 mg/day given only on Days 1, 8, 15, and 22 of each 28-day cycle for patients aged greater than 75 years). Patients in the POMALYST alone arm were allowed to add Low-dose Dex upon disease progression.
Table 7 summarizes the baseline patient and disease characteristics in Trial 1. The baseline demographics and disease characteristics were balanced and comparable between the study arms.
| POMALYST
(n=108) | POMALYST + Low-dose Dex
(n=113) |
|
|---|---|---|
| Data cutoff: 01 April 2011 | ||
|
Patient Characteristics |
||
|
Median age, years (range) |
61 (37-88) |
64 (34-88) |
|
Age distribution, n (%) | ||
|
<65 years |
65 (60.2) |
60 (53.1) |
|
≥65 years |
43 (39.8) |
53 (46.9) |
|
Sex, n (%) | ||
|
Male |
57 (52.8) |
62 (54.9) |
|
Female |
51 (47.2) |
51 (45.1) |
|
Race/ethnicity, n (%) | ||
|
White |
86 (79.6) |
92 (81.4) |
|
Black or African American |
16 (14.8) |
17 (15) |
|
All other race |
6 (5.6) |
4 (3.6) |
|
ECOG Performance, n (%) | ||
|
Status 0-1 |
95 (87.9) |
100 (88.5) |
|
Disease Characteristics |
||
|
Number of prior therapies | ||
|
Median (min, max) |
5 (2, 12) |
5 (2, 13) |
|
Prior transplant, n (%) |
82 (75.9) |
84 (74.3) |
|
Refractory to bortezomib and lenalidomide, n (%) |
64 (59.3) |
69 (61.1) |
Table 8 summarizes the analysis results of overall response rate (ORR) and duration of response (DOR), based on assessments by the Independent Review Adjudication Committee for the treatment arms in Trial 1. ORR did not differ based on type of prior antimyeloma therapy.
| a Results are prior to the addition of dexamethasone. b ORR = PR + CR per EBMT criteria. CI, confidence interval; NE, not established (the median has not yet been reached). Data cutoff: 01 April 2011 |
||
|
POMALYSTa
|
POMALYST + Low-dose Dex
|
|
|
Response |
||
|
Overall Response Rate (ORR),b n (%) |
8 (7.4) |
33 (29.2) |
|
95% CI for ORR (%) |
(3.3, 14.1) |
(21.0, 38.5) |
|
Complete Response (CR), n (%) |
0 (0.0) |
1 (0.9) |
|
Partial Response (PR), n (%) |
8 (7.4) |
32 (28.3) |
|
Duration of Response (DOR) | ||
|
Median, months |
NE |
7.4 |
|
95% CI for DOR (months) |
NE |
(5.1, 9.2) |
Trial 2
Trial 2 was a Phase 3 multi-center, randomized, open-label study, where POMALYST + Low-dose Dex therapy was compared to High-dose Dex in adult patients with relapsed and refractory MM, who had received at least two prior treatment regimens, including lenalidomide and bortezomib, and demonstrated disease progression on or within 60 days of the last therapy. Patients with creatinine clearance ≥ 45mL/min qualified for the trial. A total of 455 patients were enrolled in the trial: 302 in the POMALYST + Low-dose Dex arm and 153 in the High-dose Dex arm. Patients in the POMALYST + Low-dose Dex arm were administered 4 mg POMALYST orally on Days 1 to 21 of each 28-day cycle. Dexamethasone (40 mg) was administered once per day on Days 1, 8, 15 and 22 of a 28-day cycle. Patients > 75 years of age started treatment with 20 mg dexamethasone using the same schedule. For the High-dose Dex arm, dexamethasone (40 mg) was administered once per day on Days 1 through 4, 9 through 12, and 17 through 20 of a 28-day cycle. Patients > 75 years of age started treatment with 20 mg dexamethasone using the same schedule. Treatment continued until patients had disease progression.
Baseline patient and disease characteristics were balanced and comparable between the study arms, as summarized in Table 9. Overall, 94% of patients had disease refractory to lenalidomide, 79% had disease refractory to bortezomib and 74% had disease refractory to both lenalidomide and bortezomib.
| Data cutoff: 01March 2013 | ||
|
POMALYST + Low-dose Dex |
High-dose Dex |
|
|
(N=302) |
(N=153) |
|
|
Patient Characteristics |
||
|
Median Age, years (range) |
64 (35, 84) |
65 (35, 87) |
|
Age Distribution n (%) | ||
|
< 65 years |
158 (52) |
74 (48) |
|
≥ 65 years |
144 (48) |
79 (52) |
|
Sex n (%) | ||
|
Male |
181 (60) |
87 (57) |
|
Female |
121 (40) |
66 (43) |
|
Race/Ethnicity n (%) | ||
|
White |
244 (81) |
113 (74) |
|
Black or African American |
4 (1) |
3 (2) |
|
Asian |
4 (1) |
0 (0) |
|
Other Race |
2 (1) |
2 (1) |
|
Not Collected |
48 (16) |
35 (23) |
|
ECOG Performance n (%) | ||
|
Status 0 |
110 (36) |
36 (24) |
|
Status 1 |
138 (46) |
86 (56) |
|
Status 2 |
52 (17) |
25 (16) |
|
Status 3 |
0 (0) |
3 (2) |
|
Missing |
2 (1) |
3 (2) |
|
Disease Characteristics |
||
|
Number of Prior Therapies | ||
|
Median, (Min, Max) |
5 (2, 14) |
5 (2, 17) |
|
Prior stem cell transplant n (%) |
214 (71) |
105 (69) |
|
Refractory to bortezomib and lenalidomide n (%) |
225 (75) |
113 (74) |
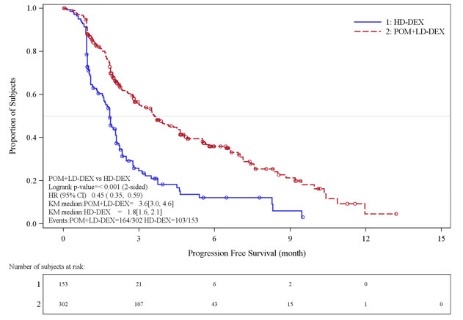
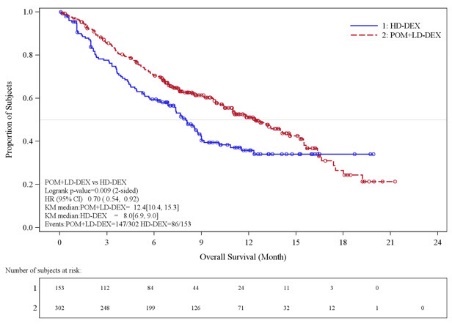
Table 10 summarizes the progression free survival (PFS) and overall response rate (ORR) based on the assessment by the Independent Review Adjudication Committee (IRAC) review at the final PFS analysis and overall survival (OS) at the OS analysis. PFS was significantly longer with POMALYST + Low-dose Dex than High-dose Dex: HR 0.45 (95% CI: 0.35-0.59 p < 0.001). OS was also significantly longer with POMALYST + Low-dose Dex than High-dose Dex: HR 0.70 (95% CI: 0.54-0.92 p = 0.009). The Kaplan-Meier curves for PFS and OS for the ITT population are provided in Figures 1 and 2, respectively.
| Note: CI=Confidence interval; HD-Dex=High dose dexamethasone; IRAC=Independent Review Adjudication Committee; LD-Dex=Low dose dexamethasone. a The median is based on Kaplan-Meier estimate. b Based on Cox proportional hazards model comparing the hazard functions associated with treatment groups, stratified by age (≤75 vs >75), diseases population (refractory to both Lenalidomide and Bortezomib vs not refractory to both drugs), and prior number of antimyeloma therapy (=2 vs >2), stratification factors for the trial. c The p-value is based on a stratified log-rank test with the same stratification factors as the above Cox model. d 53% of patients in the High-dose Dex arm subsequently received POMALYST. e Based on Cox proportional hazards model (unstratified) comparing the hazard functions associated with treatment groups. f The p-value is based on an unstratified log-rank test. g Alpha control for PFS and OS. Data cutoff: 07 Sep 2012 for PFS Data cutoff: 01 Mar 2013 for OS and ORR |
||
|
POMALYST + Low-dose Dex |
High-dose Dex |
|
|
(N=302) |
(N=153) |
|
|
Progression Free Survival Time | ||
|
Number (%) of events |
164 (54.3) |
103 (67.3) |
|
Mediana (2-sided 95% CI) (months) |
3.6 [3.0, 4.6] |
1.8 [1.6, 2.1] |
|
Hazard Ratio (Pom+LD-Dex:HD-Dex) 2-Sided 95% CIb |
0.45 [0.35, 0.59] |
|
|
Log-Rank Test 2-sided P-Valuec |
<0.001 |
|
|
Overall Survival Timed | ||
|
Number (%) of deaths |
147 (48.7) |
86 (56.2) |
|
Mediana (2-sided 95% CI) (months) |
12.4 [10.4, 15.3] |
8.0 [6.9, 9.0] |
|
Hazard Ratio (Pom+LD-Dex:HD-Dex) 2-Sided 95% CIe |
0.70 [0.54, 0.92] |
|
|
Log-Rank Test 2-sided P-Value f, g |
0.009 |
|
|
Overall Response Rate, n (%) |
71 (23.5) |
6 (3.9) |
|
Complete Response |
1 (0.3) |
0 |
|
Very Good Partial Response |
8 (2.6) |
1 (0.7) |
|
Partial Response |
62 (20.5) |
5 (3.3) |
Figure 1: Progression Free Survival Based on IRAC Review of Response by IMWG Criteria (Stratified Log Rank Test) (ITT Population)
Data cut-off: 07 Sep 2012
Figure 2: Kaplan-Meier Curve of Overall Survival (ITT Population)
Data cutoff: 01 Mar 2013
14.2 Kaposi Sarcoma
The clinical trial 12-C-0047 (NCT01495598), was an open label, single center, single arm clinical study that evaluated the safety and efficacy of POMALYST in patients with Kaposi sarcoma (KS). A total of 28 patients (18 HIV-positive, 10 HIV-negative) received POMALYST 5 mg orally once daily on Days 1 through 21 of each 28-day cycle until disease progression or unacceptable toxicity. All HIV-positive patients continued highly active antiretroviral therapy (HAART). The trial excluded patients with symptomatic pulmonary or visceral KS, history of venous or arterial thromboembolism, or procoagulant disorders. Patients received thromboprophylaxis with aspirin 81 mg once daily throughout therapy.
The median age was 52.5 years, all were male, 75% were White, and 14% Black or African American. Seventy-five percent of patients had advanced disease (T1) at the time of enrollment, 11% had ≥ 50 lesions, and 75% had received prior chemotherapy.
The major efficacy outcome measure was overall response rate (ORR), which included complete response (CR), clinical complete response (cCR), and partial response (PR). Response was assessed by the investigator according to the AIDS Clinical Trial Group (ACTG) Oncology Committee response criteria for KS. The median time to first response was 1.8 months (0.9 to 7.6). Efficacy results are presented in Table 11.
| CI: confidence interval, ORR: overall response rate, CR: complete response, PR: partial response 1 CR includes one HIV-negative patient who achieved a cCR. 2 Calculated as date of first documented response to date of first documented disease progression, receipt of new treatment or second course of treatment, or death due to any cause, whichever occurs first. Median estimate is from Kaplan-Meier analysis. 3 From Kaplan-Meier analysis. |
|||
|
All Patients
|
HIV-Positive
|
HIV-Negative
|
|
|
ORR 1, n (%) |
20 (71) |
12 (67) |
8 (80) |
|
[95% CI] |
[51, 87] |
(41, 87) |
(44, 98) |
|
CR 1, n (%) |
4 (14) |
3 (17) |
1 (10) |
|
PR, n (%) |
16 (57) |
9 (50) |
7 (70) |
|
Duration of Response, KS 2, |
12.1 |
12.5 |
10.5 |
|
Median in months [95% CI]3 |
[7.6, 16.8] |
[6.5, 24.9] |
[3.9, 24.2] |
|
Duration of Response, KS (%) | |||
|
Percent greater than 12 months |
50 |
58 |
38 |
|
Percent greater than 24 months |
20 |
17 |
25 |
16 HOW SUPPLIED/STORAGE AND HANDLING
Dark blue opaque cap and yellow opaque body, imprinted "POML" on the cap in white ink and "1 mg" on the body in black ink
|
1 mg bottles of 21 |
(NDC 59572-501-21) |
|
|
1 mg bottles of 100 |
(NDC 59572-501-00) |
Dark blue opaque cap and orange opaque body, imprinted "POML" on the cap and "2 mg" on the body in white ink
|
2 mg bottles of 21 |
(NDC 59572-502-21) |
|
|
2 mg bottles of 100 |
(NDC 59572-502-00) |
Dark blue opaque cap and green opaque body, imprinted "POML" on the cap and "3 mg" on the body in white ink
|
3 mg bottles of 21 |
(NDC 59572-503-21) |
|
|
3 mg bottles of 100 |
(NDC 59572-503-00) |
Dark blue opaque cap and blue opaque body, imprinted "POML" on the cap and "4 mg" on the body in white ink
|
4 mg bottles of 21 |
(NDC 59572-504-21) |
|
|
4 mg bottles of 100 |
(NDC 59572-504-00) |
Store at 20°C-25°C (68°F-77°F); excursions permitted to 15°C-30°C (59°F-86°F) [see USP Controlled Room Temperature].
Care should be exercised in handling of POMALYST. Do not open or crush POMALYST capsules. If powder from POMALYST contacts the skin, wash the skin immediately and thoroughly with soap and water. If POMALYST contacts the mucous membranes, flush thoroughly with water.
Follow procedures for proper handling and disposal of hazardous drugs. 1
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Embryo-Fetal Toxicity
Advise patients that POMALYST is contraindicated in pregnancy [see Contraindications (4)]. POMALYST is a thalidomide analogue and may cause serious birth defects or death to a developing baby [see Warnings and Precautions (5.1) and Use in Specific Populations (8.1)].
- •
- Advise females of reproductive potential that they must avoid pregnancy while taking POMALYST and for at least 4 weeks after completing therapy.
- •
- Initiate POMALYST treatment in females of reproductive potential only following a negative pregnancy test.
- •
- Advise females of reproductive potential of the importance of monthly pregnancy tests and the need to use 2 different forms of contraception, including at least 1 highly effective form, simultaneously during POMALYST therapy, during dose interruptions, and for 4 weeks after she has completely finished taking POMALYST. Highly effective forms of contraception other than tubal ligation include IUD and hormonal (birth control pills, injections, patch, or implants) and a partner's vasectomy. Additional effective contraceptive methods include latex or synthetic condom, diaphragm, and cervical cap.
- •
- Instruct patient to immediately stop taking POMALYST and contact her healthcare provider if she becomes pregnant while taking this drug, if she misses her menstrual period or experiences unusual menstrual bleeding, if she stops taking birth control, or if she thinks FOR ANY REASON that she may be pregnant.
- •
- Advise patient that if her healthcare provider is not available, she should call the REMS Call Center at 1-888-423-5436 [see Warnings and Precautions (5.1) and Use in Specific Populations (8.3)].
- •
- Advise males to always use a latex or synthetic condom during any sexual contact with females of reproductive potential while taking POMALYST and for up to 4 weeks after discontinuing POMALYST, even if they have undergone a successful vasectomy.
- •
- Advise male patients taking POMALYST that they must not donate sperm [see Warnings and Precautions (5.1) and Use in Specific Populations (8.3)].
- •
- All patients must be instructed to not donate blood while taking POMALYST and for 4 weeks following discontinuation of POMALYST [see Warnings and Precautions (5.1)].
POMALYST REMS Program
Because of the risk of embryo-fetal toxicity, POMALYST is only available through a restricted program called POMALYST REMS [see Warnings and Precautions (5.2)].
- •
- Patients must sign a Patient-Physician Agreement Form and comply with the requirements to receive POMALYST. In particular, females of reproductive potential must comply with the pregnancy testing, contraception requirements, and participate in monthly telephone surveys. Males must comply with the contraception requirements [see Use in Specific Populations (8.3)].
- •
- POMALYST is available only from pharmacies that are certified in POMALYST REMS program. Provide patients with the telephone number and website for information on how to obtain the product.
Pregnancy Exposure Registry
Inform females that there is a Pregnancy Exposure Registry that monitors pregnancy outcomes in females exposed to POMALYST during pregnancy and that they can contact the Pregnancy Exposure Registry by calling 1-888-423-5436 [see Use in Specific Populations (8.1)].
Venous and Arterial Thromboembolism
Inform patients of the risk of developing DVT, PE, MI, and stroke and to report immediately any signs and symptoms suggestive of these events for evaluation [see Warnings and Precautions (5.3)].
Hematologic Toxicities
Inform patients on the risks of developing neutropenia, thrombocytopenia, and anemia and the need to report signs and symptoms associated with these events to their healthcare provider for further evaluation [see Warnings and Precautions (5.5)].
Hepatotoxicity
Inform patients on the risks of developing hepatotoxicity, including hepatic failure and death, and to report signs and symptoms associated with these events to their healthcare provider for evaluation [see Warnings and Precautions (5.6)].
Severe Cutaneous Reactions
Inform patients of the potential risk for severe skin reactions such as SJS, TEN and DRESS and to report any signs and symptoms associated with these reactions to their healthcare provider for evaluation [see Warnings and Precautions (5.7)].
Dizziness and Confusional State
Inform patients of the potential risk of dizziness and confusional state with the drug, to avoid situations where dizziness or confusional state may be a problem, and not to take other medications that may cause dizziness or confusional state without adequate medical advice [see Warnings and Precautions (5.8)].
Neuropathy
Inform patients of the risk of neuropathy and to report the signs and symptoms associated with these events to their healthcare provider for further evaluation [see Warnings and Precautions (5.9)].
Second Primary Malignancies
Inform the patient that the potential risk of developing acute myelogenous leukemia during treatment with POMALYST is unknown [see Warnings and Precautions (5.10)].
Tumor Lysis Syndrome
Inform patients of the potential risk of tumor lysis syndrome and to report any signs and symptoms associated with this event to their healthcare provider for evaluation [see Warnings and Precautions (5.11)].
Hypersensitivity
Inform patients of the potential for severe hypersensitivity reactions such as angioedema and anaphylaxis to POMALYST. Instruct patients to contact their healthcare provider right away for any signs and symptoms of these reactions. Advise patients to seek emergency medical attention for signs or symptoms of severe hypersensitivity reactions [see Warnings and Precautions (5.12)].
Smoking Tobacco
Advise patients that smoking tobacco may reduce the efficacy of POMALYST [see Use in Specific Populations (8.8) and Clinical Pharmacology (12.3)].
Dosing Instructions
Inform patients on how to take POMALYST [see Dosage and Administration (2.2, 2.3, 2.9)]
- •
- POMALYST should be taken once daily at about the same time each day.
- •
- Patients on hemodialysis should take POMALYST following hemodialysis, on hemodialysis days.
- •
- POMALYST may be taken with or without food.
- •
- The capsules should not be opened, broken, or chewed. POMALYST should be swallowed whole with water.
- •
- Instruct patients that if they miss a dose of POMALYST, they may still take it up to 12 hours after the time they would normally take it. If more than 12 hours have elapsed, they should be instructed to skip the dose for that day. The next day, they should take POMALYST at the usual time. Warn patients not to take 2 doses to make up for the one that they missed.
Marketed by: Bristol-Myers Squibb Company
Princeton, NJ 08543 USA
POMALYST® and POMALYST REMS® are trademarks of Celgene Corporation, a Bristol-Myers Squibb company.
POMPI.016/MG.014
|
This Medication Guide has been approved by the U.S. Food and Drug Administration. |
Revised: March 2023 |
|||
|
MEDICATION GUIDE
|
||||
|
What is the most important information I should know about POMALYST? |
||||
|
Before you begin taking POMALYST, you must read and agree to all of the instructions in the POMALYST REMS® program. For more information, call 1-888-423-5436 or go to www.pomalystrems.com. Before prescribing POMALYST, your healthcare provider will explain the POMALYST REMS program to you and have you sign the Patient-Physician Agreement Form. |
||||
|
POMALYST can cause serious side effects including: |
||||
|
||||
|
What is POMALYST? |
||||
|
POMALYST is a prescription medicine used to treat adults with: |
||||
|
||||
|
It is not known if POMALYST is safe and effective in children. |
||||
|
Who should not take POMALYST? |
||||
|
Do not take POMALYST if you: |
||||
|
||||
|
What should I tell my healthcare provider before taking POMALYST? |
||||
|
Before you take POMALYST, tell your healthcare provider if you: |
||||
|
||||
|
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. POMALYST and other medicines may affect each other, causing serious side effects. Talk with your healthcare provider before taking any new medicines. |
||||
|
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist. |
||||
|
How should I take POMALYST?
|
||||
|
What should I avoid while taking POMALYST? |
||||
|
||||
|
What are the possible side effects of POMALYST? |
||||
|
POMALYST can cause serious side effects, including: |
||||
|
||||
|
|
|||
|
||||
|
|
|
||
|
Get emergency medical help right away if you develop any of the following signs or symptoms during treatment with POMALYST: |
||||
|
|
|||
|
||||
|
Your healthcare provider may tell you to stop taking POMALYST if you develop certain serious side effects during treatment. |
||||
|
The most common side effects of POMALYST in people with Multiple Myeloma include: |
||||
|
|
|
||
|
The most common side effects of POMALYST in people with KS include: |
||||
|
|
|||
|
These are not all the possible side effects of POMALYST. |
||||
|
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
||||
|
How should I store POMALYST? |
||||
|
||||
|
Keep POMALYST and all medicines out of the reach of children. |
||||
|
General information about the safe and effective use of POMALYST. |
||||
|
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not take POMALYST for conditions for which it was not prescribed. Do not give POMALYST to other people, even if they have the same symptoms you have. It may harm them and may cause birth defects. |
||||
|
If you would like more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about POMALYST that is written for health professionals. |
||||
|
For more information, call 1-888-423-5436 or go to www.pomalystrems.com. |
||||
|
What are the ingredients in POMALYST? |
||||
|
Active ingredient: pomalidomide |
||||
|
Inactive ingredients: mannitol, pregelatinized starch, and sodium stearyl fumarate. |
||||
|
The 1-mg capsule shell contains gelatin, titanium dioxide, FD&C blue 2, yellow iron oxide, white ink, and black ink. |
||||
|
Marketed by: Bristol-Myers Squibb Company, Princeton, NJ 08543 USA |
||||
POMALYST 1 mg Representative Packaging
NDC 59572-501-21
Pomalyst®
(pomalidomide) capsules
1 mg
WARNING: POTENTIAL FOR HUMAN BIRTH DEFECTS.
Rx only
21 Capsules
Bristol Myers Squibb
POMALYST 2 mg Representative Packaging
NDC 59572-502-21
Pomalyst®
(pomalidomide) capsules
2 mg
WARNING: POTENTIAL FOR HUMAN BIRTH DEFECTS.
Rx only
21 Capsules
Bristol Myers Squibb