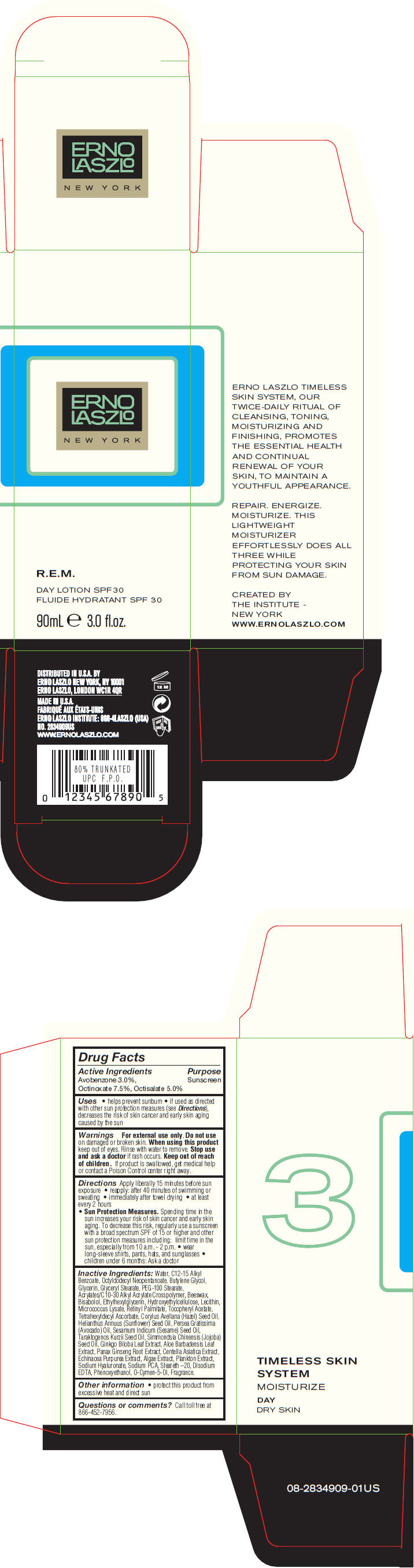
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
Directions
Apply liberally 15 minutes before sun exposure
- reapply: after 40 minutes of swimming or sweating
- immediately after towel drying
- at least every 2 hours
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including: limit time in the sun, especially from 10 a.m. - 2 p.m.
- wear long-sleeve shirts, pants, hats, and sunglasses
- children under 6 months: Ask a doctor
Inactive Ingredients
Water, C12-15 Alkyl Benzoate, Octyldodecyl Neopentanoate, Butylene Glycol, Glycerin, Glyceryl Stearate, PEG-100 Stearate, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Beeswax, Bisabolol, Ethylhexylglycerin, Hydroxyethylcellulose, Lecithin, Micrococcus Lysate, Retinyl Palmitate, Tocopheryl Acetate, Tetrahexyldecyl Ascorbate, Corylus Avellana (Hazel) Seed Oil, Helianthus Annuus (Sunflower) Seed Oil, Persea Gratissima (Avocado) Oil, Sesamum Indicum (Sesame) Seed Oil, Taraktogenos Kurzii Seed Oil, Simmondsia Chinensis (Jojoba) Seed Oil, Ginkgo Biloba Leaf Extract, Aloe Barbadensis Leaf Extract, Panax Ginseng Root Extract, Centella Asiatica Extract, Echinacea Purpurea Extract, Algae Extract, Plankton Extract, Sodium Hyaluronate, Sodium PCA, Steareth –20, Disodium EDTA, Phenoxyethanol, O-Cymen-5-Ol, Fragrance.