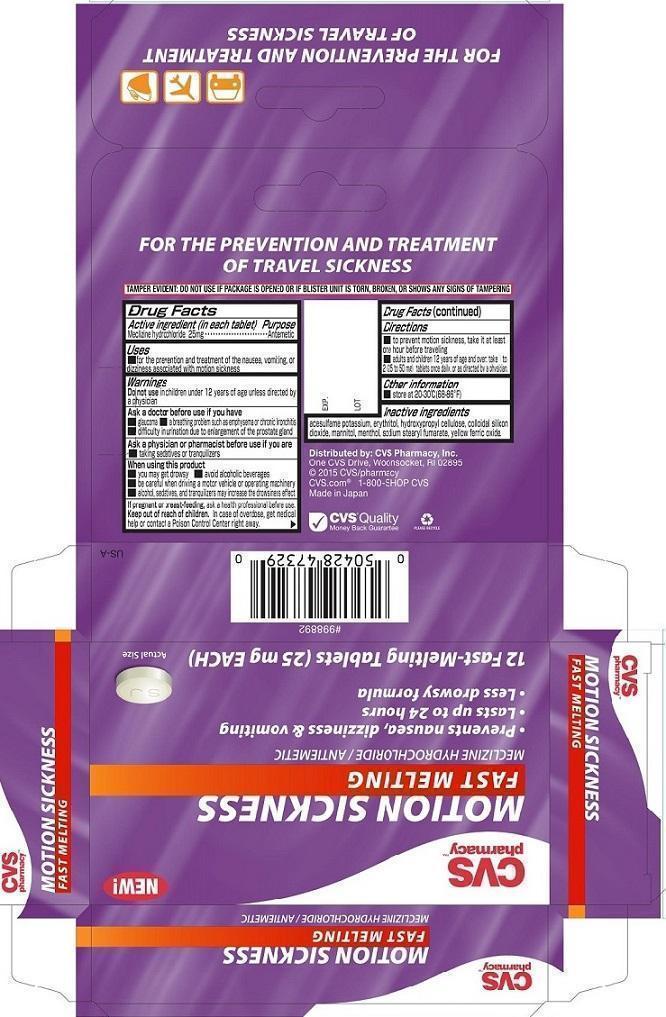
CVS MOTION SICKNESS FAST MELTING- meclizine hydrochloride tablet, orally disintegrating
CVS Pharmacy, Inc.
----------
Active ingredient (in each tablet)
Meclizine hydrochloride 25mg
Purpose
Meclizine hydrochloride Antiemetic
Uses
■ For the prevention and treatment of the nausea, vomiting, or dizziness associated with motion sickness.
Warnings
Do not use in children under 12 years of age unless directed by a physician
Ask a doctor before use if you have
■ glaucoma ■ a breathing problem such as emphysema or chronic bronchitis
■ difficulty in urination due to enlargement of the prostate gland
Ask a physician or pharmacist before use if you are
■ taking sedatives or tranquilizers
When using this product
■ you may get drowsy ■ avoid alcoholic beverages
■ be careful when driving a motor vehicle or operating machinery
■ alcohol, sedatives, and tranquilizers may increase the drowsiness effect
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
To prevent motion sickness take it at least one hour before traveling
Adults and children 12 years of age and over: take 1 to 2 (25 to 50 mg) tablets once daily, or as directed by a physician.
Other information
■ Store at 20-30°C (68-86°F)
Inactive ingredients
acesulfame potassium, erythritol, hydroxypropyl cellulose, colloidal silicon dioxide, mannitol, menthol, sodium stearyl fumarate, yellow ferric oxide.
CVS Pharmacy, Inc.