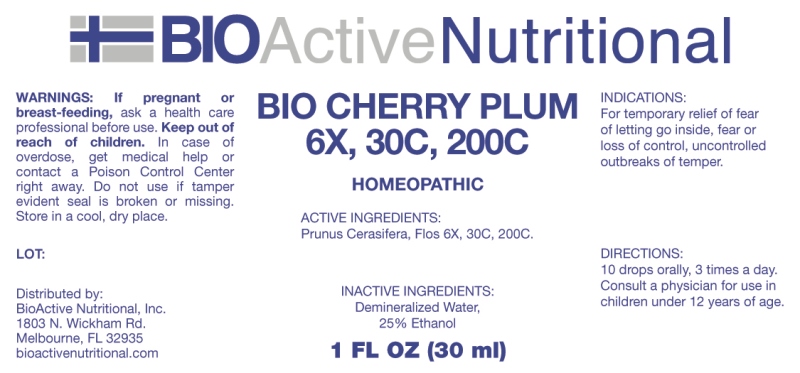
INDICATIONS:
For temporary relief of fear of letting go inside, fear or loss of control, uncontrolled outbreaks of temper.
WARNINGS:
If pregnant or breast-feeding, ask a health care professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Do not use if tamper evident seal is broken or missing.
Store in cool, dry place.
DIRECTIONS:
10 drops orally, 3 times a day. Consult a physician for use in children under 12 years of age.
KEEP OUT OF REACH OF CHILDREN:
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
INDICATIONS:
For temporary relief of fear of letting go inside, fear or loss of control, uncontrolled outbreaks of temper.