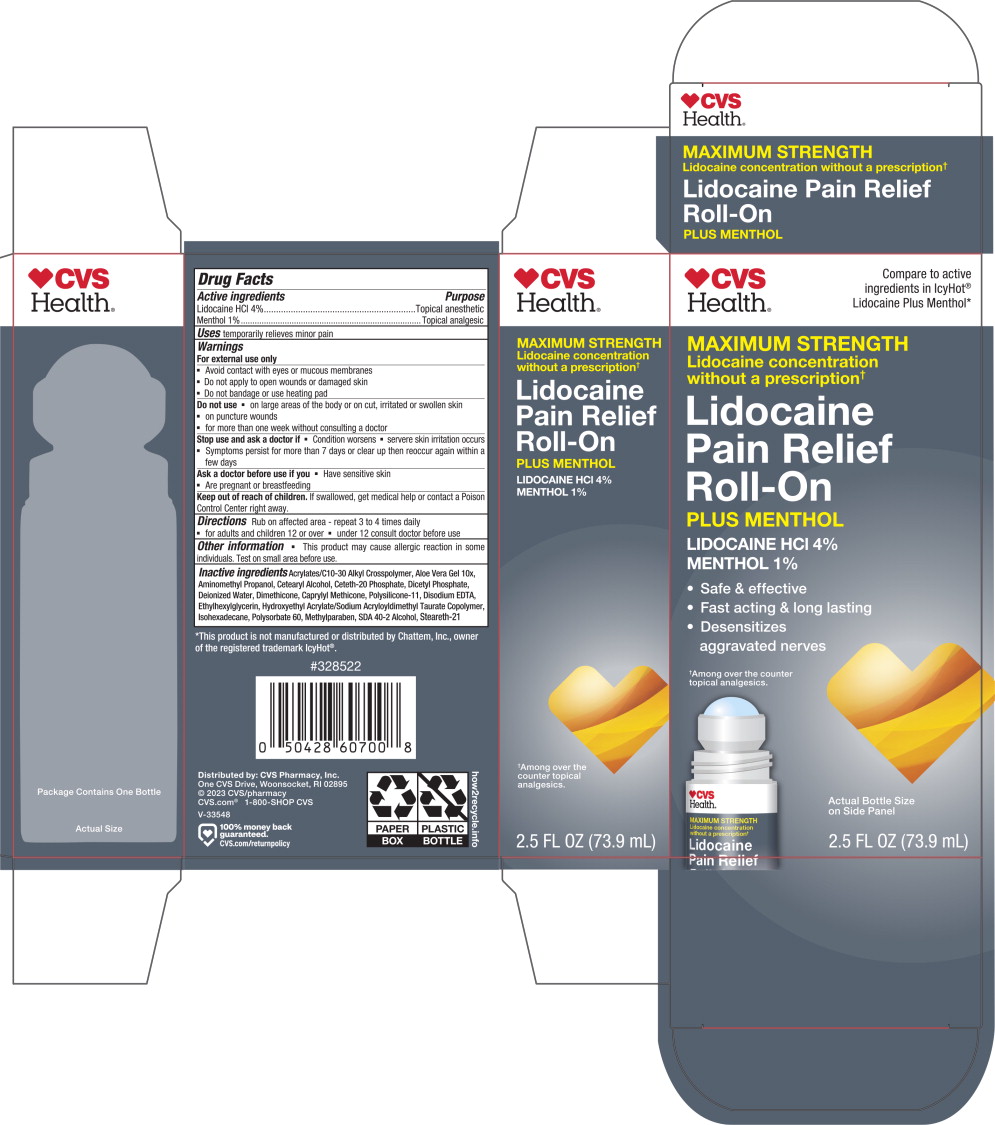
Warnings
For external use only
- Avoid contact with eyes or mucous membranes
- Do not apply to open wounds or damaged skin
- Do not bandage or use heating pad
Do not use
- on large areas of the body or on cut, irritated or swollen skin
- on puncture wounds
- for more than one week without consulting a doctor
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
Rub on affected area - repeat 3 to 4 times daily
- for adults and children 12 or over
- under 12 consult doctor before use
Other information
- This product may cause allergic reaction in some individuals. Test on small area before use.
Inactive ingredients
Acrylates/C10-30 Alkyl Crosspolymer, Aloe Vera Gel 10x, Aminomethyl Propanol, Cetearyl Alcohol, Ceteth-20 Phosphate, Dicetyl Phosphate, Deionized Water, Dimethicone, Caprylyl Methicone, Polysilicone-11, Disodium EDTA, Ethylhexylglycerin, Hydroxyethyl Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Isohexadecane, Polysorbate 60, Methylparaben, SDA 40-2 Alcohol, Steareth-21
Principal Display Panel – 73.9 mL Carton Label
CVS
Health®
Compare to active
ingredients in IcyHot®
Lidocaine Plus Menthol*
MAXIMUM STRENGTH
Lidocaine concentration
without a prescription✝
Lidocaine
Pain Relief
Roll-On
PLUS MENTHOL
LIDOCAINE HCl 4%
MENTHOL 1%
- Safe & effective
- Fast acting & long lasting
-
Desensitizes
aggravated nerves
✝Among over the counter
topical analgesics.
Actual Bottle Size
on Side Panel
2.5 FL OZ (73.9 mL)