PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
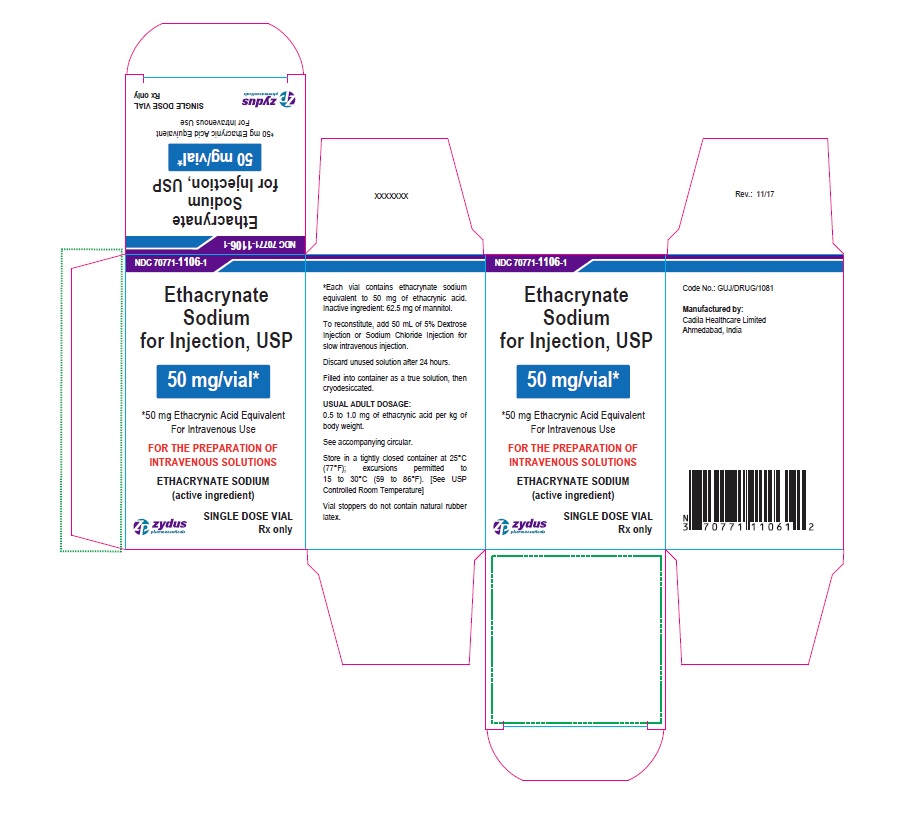
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 50 MG SINGLE DOSE VIAL CONTAINER LABEL
NDC 70771-1106-1
Ethacrynate Sodium for Injection, USP
50 mg/vial*
*50 mg Ethacrynic Acid Equivalent
For Intravenous Use
SINGLE DOSE VIAL
Rx only
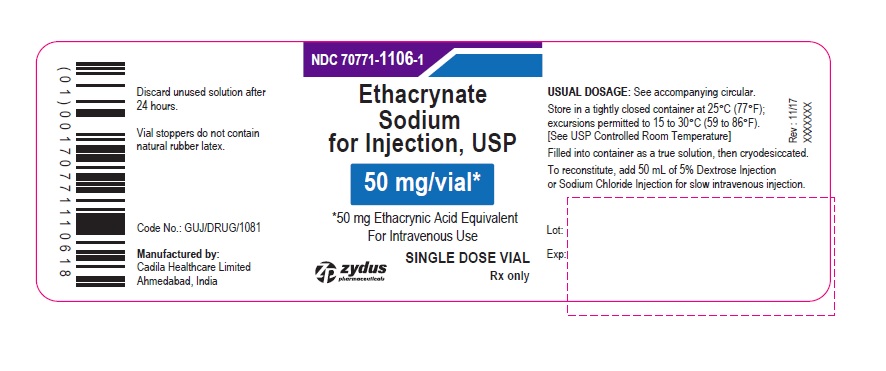
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 50 MG SINGLE DOSE VIAL CARTON LABEL
NDC 70771-1106-1
Ethacrynate Sodium for Injection, USP
50 mg/vial*
*50 mg Ethacrynic Acid Equivalent
For Intravenous Use
FOR THE PREPARATION OF INTRAVENOUS SOLUTIONS
ETHACRYNATE SODIUM (active ingredient)
SINGLE DOSE VIAL
Rx only