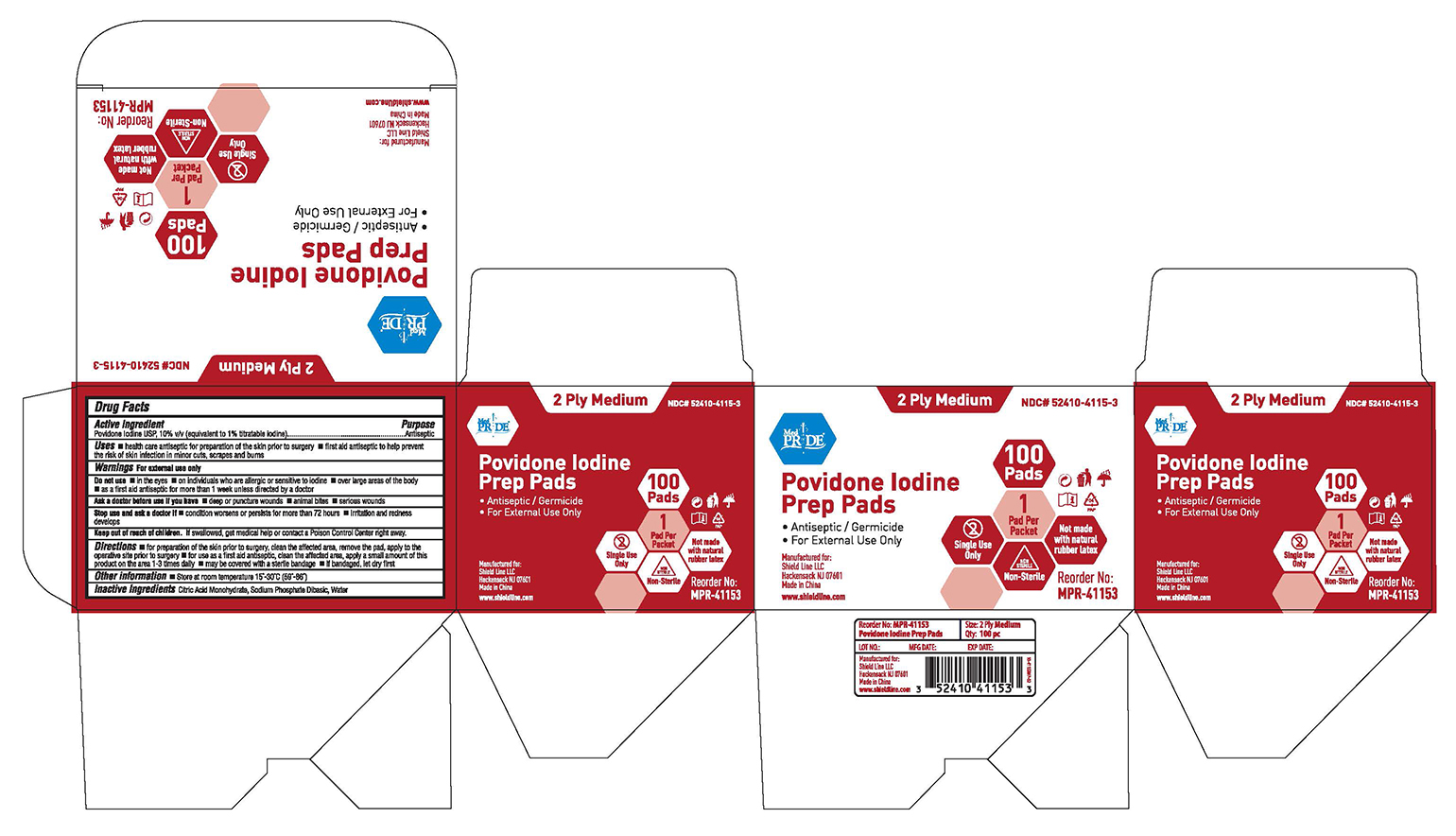
Uses
■ health care antiseptic for preparation of the skin prior to surgery
■ first aid antiseptic to help prevent the risk of skin infection in minor cuts, scrapes and burns
Do not use
■ in the eyes
■ on individuals who are allergic or sensitive to iodine
■ over large areas of the body
■ as first aid antiseptic for more than 1 week unless directed by doctor
Stop use and ask a doctor if
■ condition worsens or persists fro more than 72 hours ■ irritation and rednesss develops
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
■ for preparation of the skin prior to surgery, clean the affected area, remove the pad, apply to the operative site prior to surgery
■ for use as a first aid antiseptic, clean the affected area, apply a small amount of this product on the area 1-3 times daily ■ may be covered with a sterile bandage ■ if bandaged, let dry first