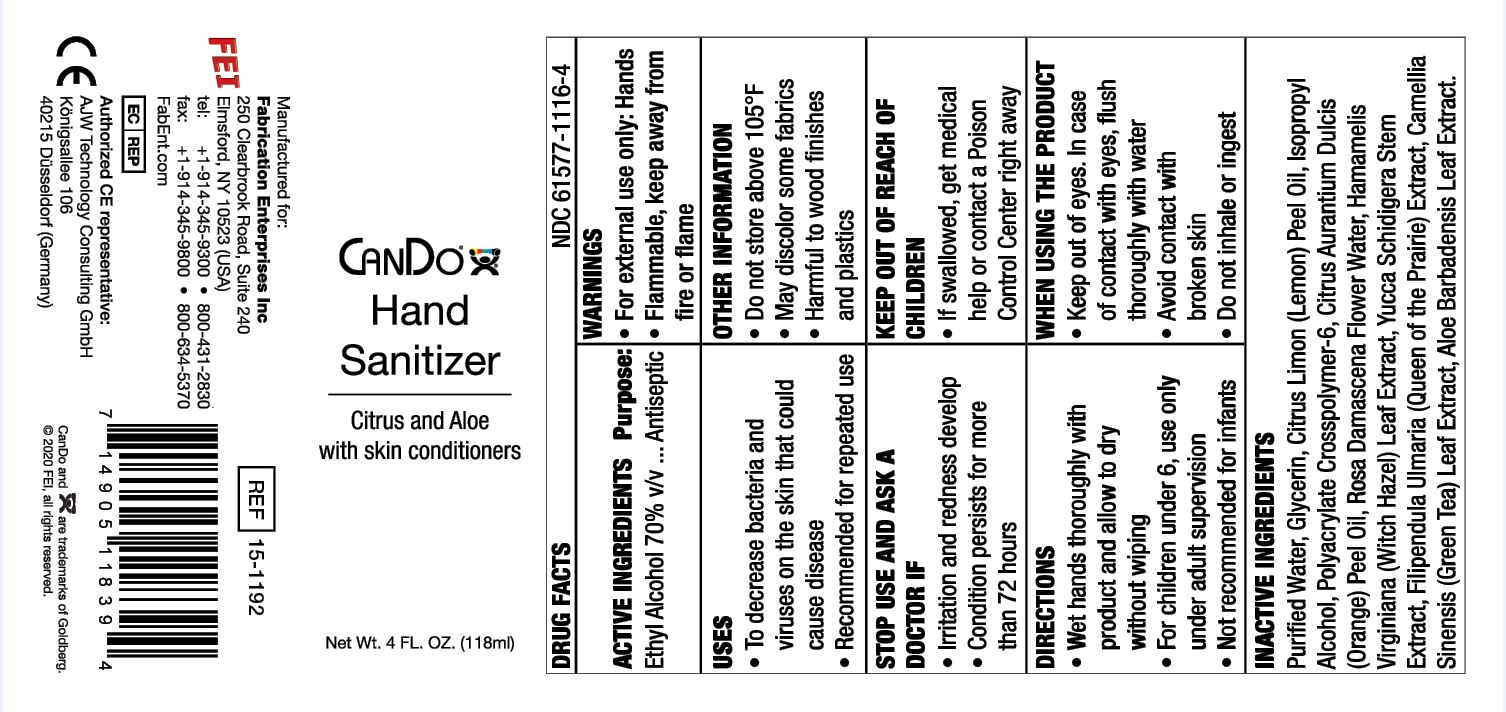
Uses
To decrease bacteria and viruses on the skin that could cause disease. Recommended for repeated use.
Do not use
When using this product, do not use in or near the eyes. In case of contact, rinse eyes thoroughly with water.
When Using
Keep out of the eyes. In case of contact with eyes, flush thoroughly with water. Avoid contact with broken skin. Do not inhale or ingest
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away. For children under 6, use only under adult supervision. Not recommended for infants.
Other information
Do not store above 105F. May discolor some fabrics. Harmful to wood finishes and plastics.
Inactive ingredients
glycerin, purified water, isopropyl alcohol, polyacrylate crosspolymer-6, rosa damascena flower water, hamamelis virginiana (witch hazel) leaf extract, aloe barbadensis leaf juice, citrus aurantium dulcis (orange) peel oil, citrus limon (lemon) peel, oil, yucca schidigera stem extract, filipendula rubra extract, camellia sinensis (green tea) leaf extract.