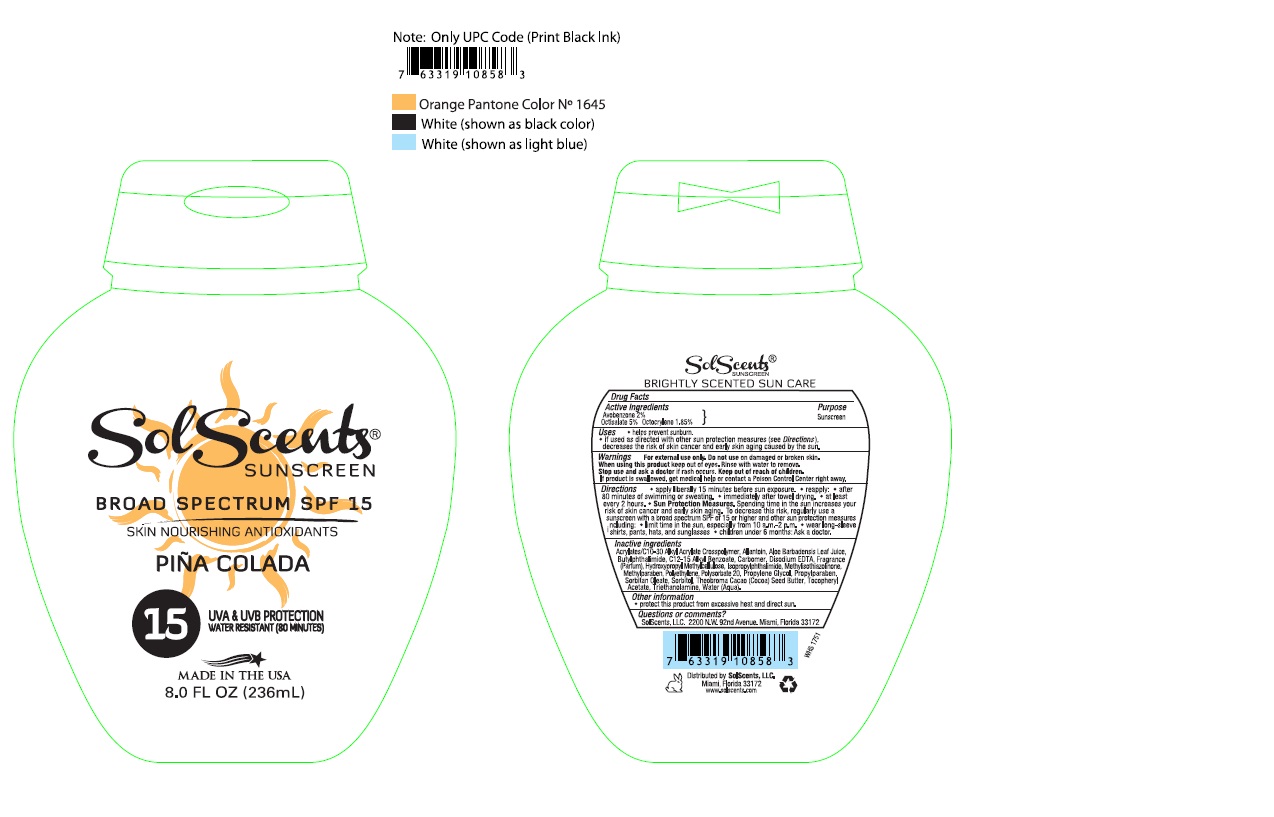
Uses
- helps prevent sunburn.
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
Directions
- apply liberally 15 minutes before sun exposure.
- reapply:
- after 80 minutes of swimming or sweating.
- immediately after towel drying.
- at least every 2 hours.
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. – 2 p.m.
- wear long-sleeve shirts, pants, hats, and sunglasses.
- children under 6 months: Ask a doctor.
Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Allantoin, Aloe Barbadensis Leaf Juice, Butylphthalimide, C12-15 Alkyl Benzoate, Carbomer, Disodium EDTA, Fragrance (Parfum), Hydroxypropyl Methylcellulose, Isopropylphthalimide, Methylisothiazolinone, Methylparaben, Polyethylene, Polysorbate 20, Propylene Glycol, Propylparaben, Sorbitan Oleate, Sorbitol, Theobroma Cacao (Cocoa) Seed Butter, Tocopheryl Acetate, Triethanolamine, Water (Aqua).