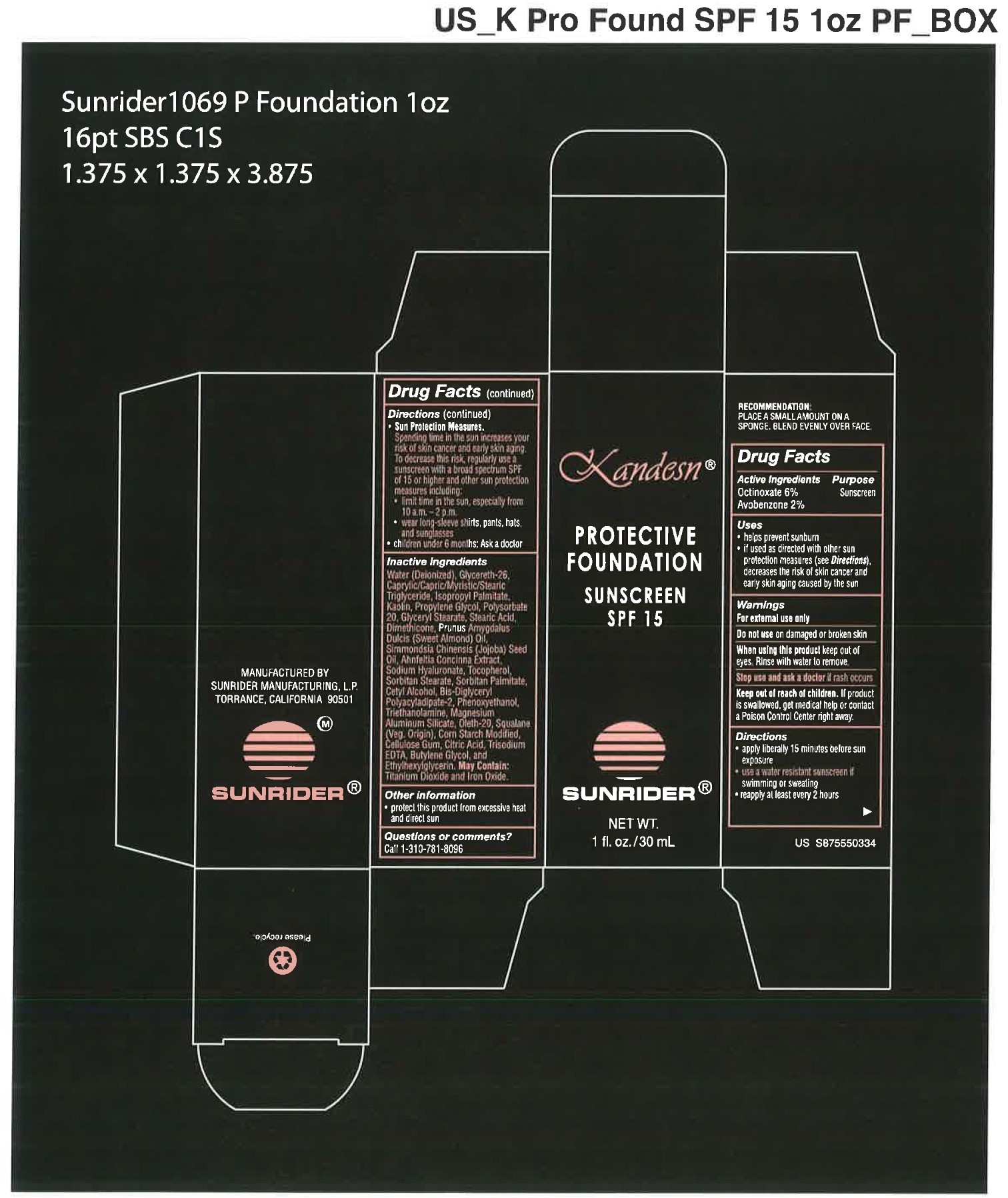
USES:
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreased the risk of skin cancer and early skin aging caused by the sun.
RECOMMENDATION: PLACE A SMALL AMOUNT ON A SPONGE, BLEND EVENLY OVER FACE
DIRECTIONS:
- apply liberally 15 minutes before sun exposure
- use a water resistant sunscreen if swimming or sweating
- reapply at least every 2 hours
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin againg. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including:
- limit time in the sun, expecially from 10 a.m. - 2 p.m.
- wear long-sleeve shirts, pants, hats, and sunglasses
- Children under 6 months: Ask a doctor
OTHER INGREDIENTS:
Water (Deionized)
Glycereth-26
Caprylic/Capric/Myristic/Stearic Triglyceride
Isopropyl Palmitate
Kaolin
Propylene Glycol
Polysorbate 20
Glyceryl Stearate
Stearic Acid (Veg. Origin)
Dimethicone
Prunus Amygdalus Dulcis (Sweet Almond) Oil
Simmondsia Chinensis (Jojoba) Seed Oil
Ahnfeltia Concinna Extract
Sodium Hyaluronate
Tocopherol
Sorbitan Stearate
Sorbitan Palmitate
Cetyl Alcohol
Bis-Diglyceryl Polyacyladipate-2
Phenoxyethanol
Triethanolamine
Magnesium Aluminum Silicate
Oleth-20
Squalane (Veg. Origin)
Corn Starch Modified
Cellulose Gum
Citric Acid
Trisodium EDTA
Butylene Glycol
Ethylhexylglycerin
WARNINGS:
FOR EXTERNAL USE ONLY
Do not use on damaged or broken skin
When using this product keep oout of eyes. Rinse with water to remove.
Sto use and ask a doctor if rash occurs.
Keep out of reach of children. If product is swallowed, get medical help or contact a Poison Control Center right away.