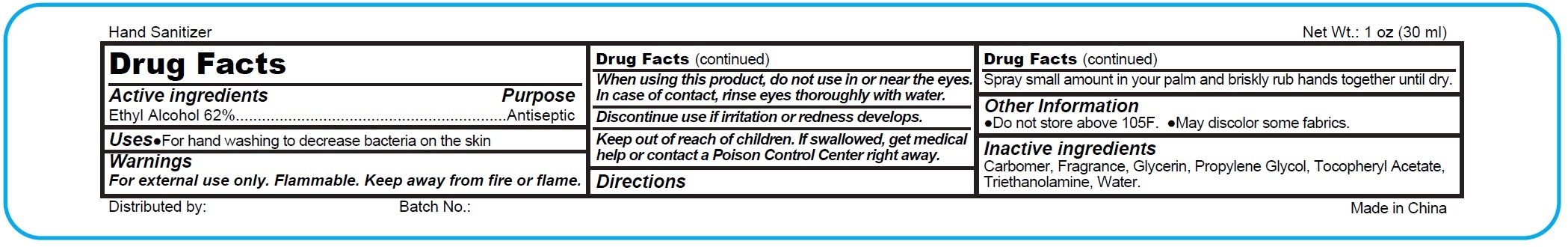
Active Ingredients
Ethyl Alcohol 62%
Uses:
Hand sanitizer to help decrease bacteria on the skin. When water, soap & towel are not available. Recommended for repeated use.
Warnings:
For external use only.
Flammable. Keep away from fire or flame.
Do not apply around eyes, Do not use in ears & mouth.
When using this product,
avoid contact with eyes. In case of contact flush eyes with water.
Stop use and ask a doctor
if redness or irritation develop and persist for more than 72 hours.
Keep out of reach of children.
Children must be supervised in use of this product.
Directions:
Squits as needed into your palms and thoroughly spread on both hands. Rub into skin until dry
Other Information:
Store at 20° C (68° to 77°F). May discolor fabrics
Inactive Ingredients
water, triethanolamine, glycerin, propylene glycol, tocopheryl acetate, carbomer, fragrance.
Package Labeling:

Package Labeling:70412-119-30
Zhejiang Ayan Biotech Co.,Ltd.

