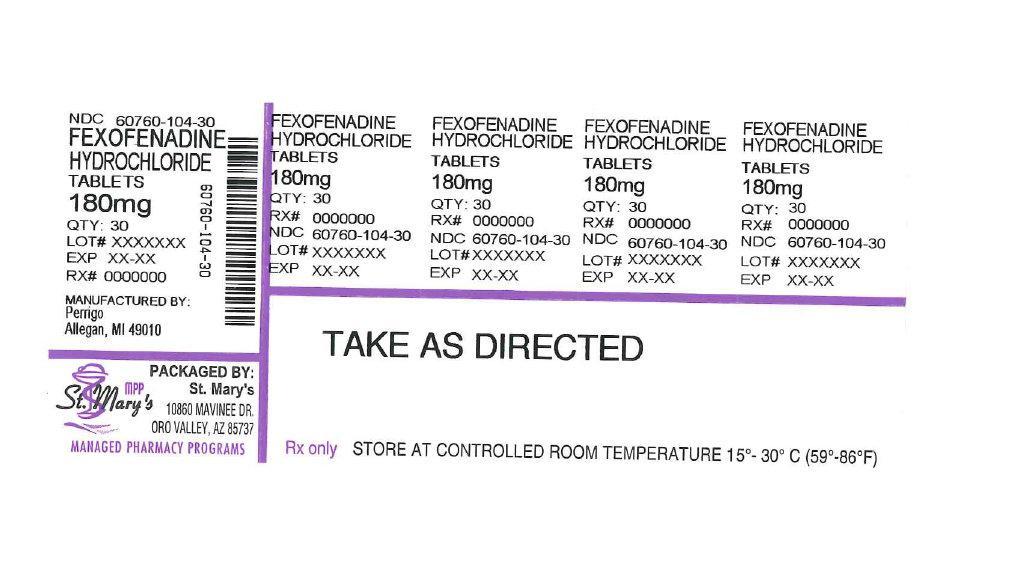
FEXOFENADINE HYDROCHLORIDE- fexofenadine hydrochloride tablet, film coated
St Marys Medical Park Pharmacy
----------
Uses
temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- itchy, watery eyes
- sneezing
- itching of the nose or throat
Warnings
Ask a doctor before use if you have
kidney disease. Your doctor should determine if you need a different dose.
When using this product
- do not take more than directed
- do not take at the same time as aluminum or magnesium antacids
- do not take with fruit juices (see Directions)
Directions
| adults and children 12 years of age and over | take one 180 mg tablet with water once a day; do not take more than 1 tablet in 24 hours |
| children under 12 years of age | do not use |
| adults 65 years of age and older | ask a doctor |
| consumers with kidney disease | ask a doctor |
Other information
- do not use if printed foil under cap is broken or missing
- store at 20°-25°C (68°-77°F)
- protect from excessive moisture
- this product meets the requirements of USP Dissolution Test 3
| FEXOFENADINE HYDROCHLORIDE
fexofenadine hydrochloride tablet, film coated |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - St Marys Medical Park Pharmacy (063050751) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| St Marys Medical Park Pharmacy | 063050751 | relabel(60760-104) , repack(60760-104) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Teva Pharmaceutical Industries Ltd. | 533065814 | manufacture(60760-104) | |
Revised: 1/2018
Document Id: 61d15588-b4b4-1667-e053-2a91aa0a1f3e
Set id: 27da5da7-3f3c-4c19-b3b8-5a4ba58fa711
Version: 2
Effective Time: 20180102
St Marys Medical Park Pharmacy