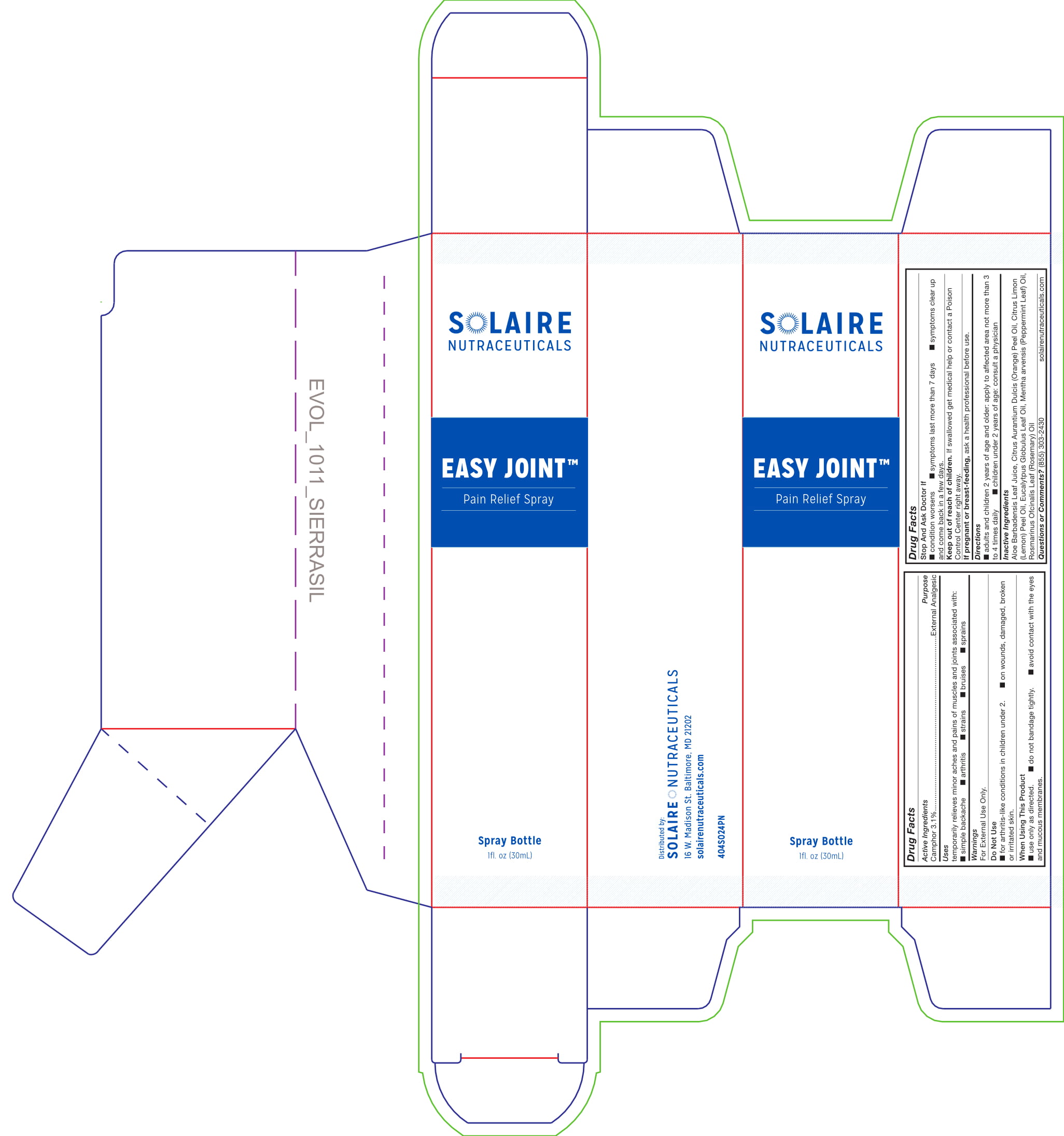
temporarily relieves minor aches and pains of muscles and joints associated with:
- simple backache
- arthritis
- strains
- bruises
- sprains
For external use only.
Do Not Use
- for arthritis-like conditions in children under 2.
- on wounds, damaged, broken or irritated skin
When Using This Product
- use only as directed.
- do not bandage tightly.
- avoid contact with eyes or mucous membranes.
Stop And Ask Doctor If
- condition worsens
- symptoms last more than 7 days
- symptoms clear up and com back in a few days
- adults and children 2 yrs of age and older: apply to affected area not more than 3 to 4 times daily
- children under 2 yrs of age: consult a physician
Aloe Barbadensis Leaf Juice, Citrus Aurantium Dulcis (Orange) Peel Oil, Citrus Limon (Lemon) Peel Oil, Eucalyptus Globulus Leaf Oil, Mentha arvensis (Peppermint) Oil, Rosmarinus Officinalis Leaf (Rosemary) Oil