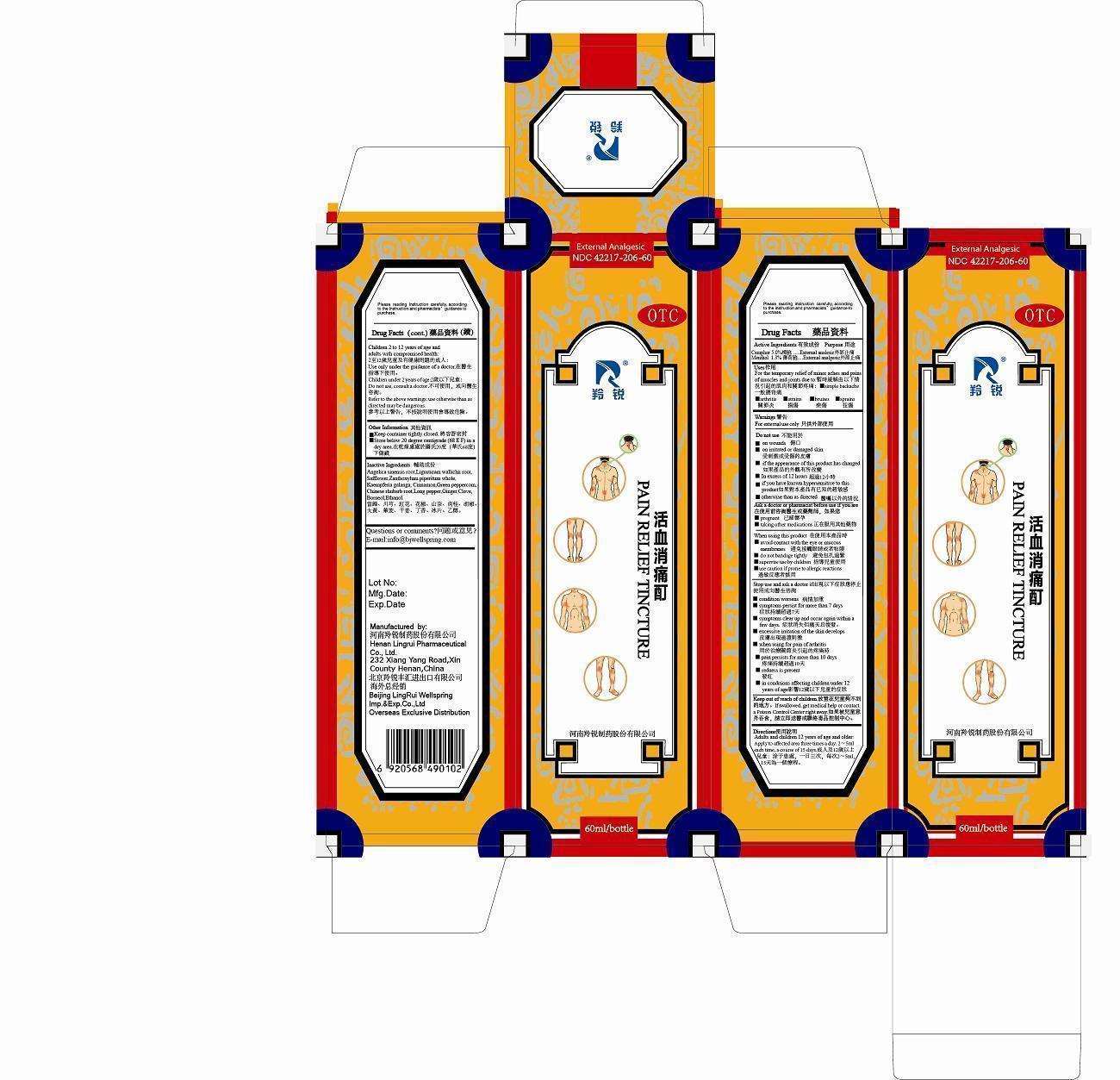
Uses
For the temporary relief of minor aches and pains of muscles and joints due to:
simple backache
arthritis
strains
bruises
sprains
Do not use
on wounds
on irritated or damaged skin
if the appearance of this product has changed
in excess of 12 hours
if you have known hypersensitive to this product
otherwise than as directed
WHEN USING THIS PRODUCT
avoid contact with the eye or mucous membranes
do not bandage tightly
supervise use by children
use caution if prone to allergic reactions
STOP USE AND ASK A DOCTOR IF
condition worsens
symptoms persist for more than 7 days
symptoms clear up and occur again within a few days
excessive irritation of the skin develops
when using for pain of arthritis
pain persists for more than 10 days
redness is present
in conditions affecting children under 12 years of age
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
Adults and children 12 years of age and older: Apply to affected area three times a day, 2 - 5ml each time, a course of 15 days.
Children 2 to 12 years of age and adults with compromised health: Use only under the guidance of a doctor.
Children under 2 years of age: Do not use, consult a doctor.
Refer to the above warnings: use otherwise than as directed may be dangerous.