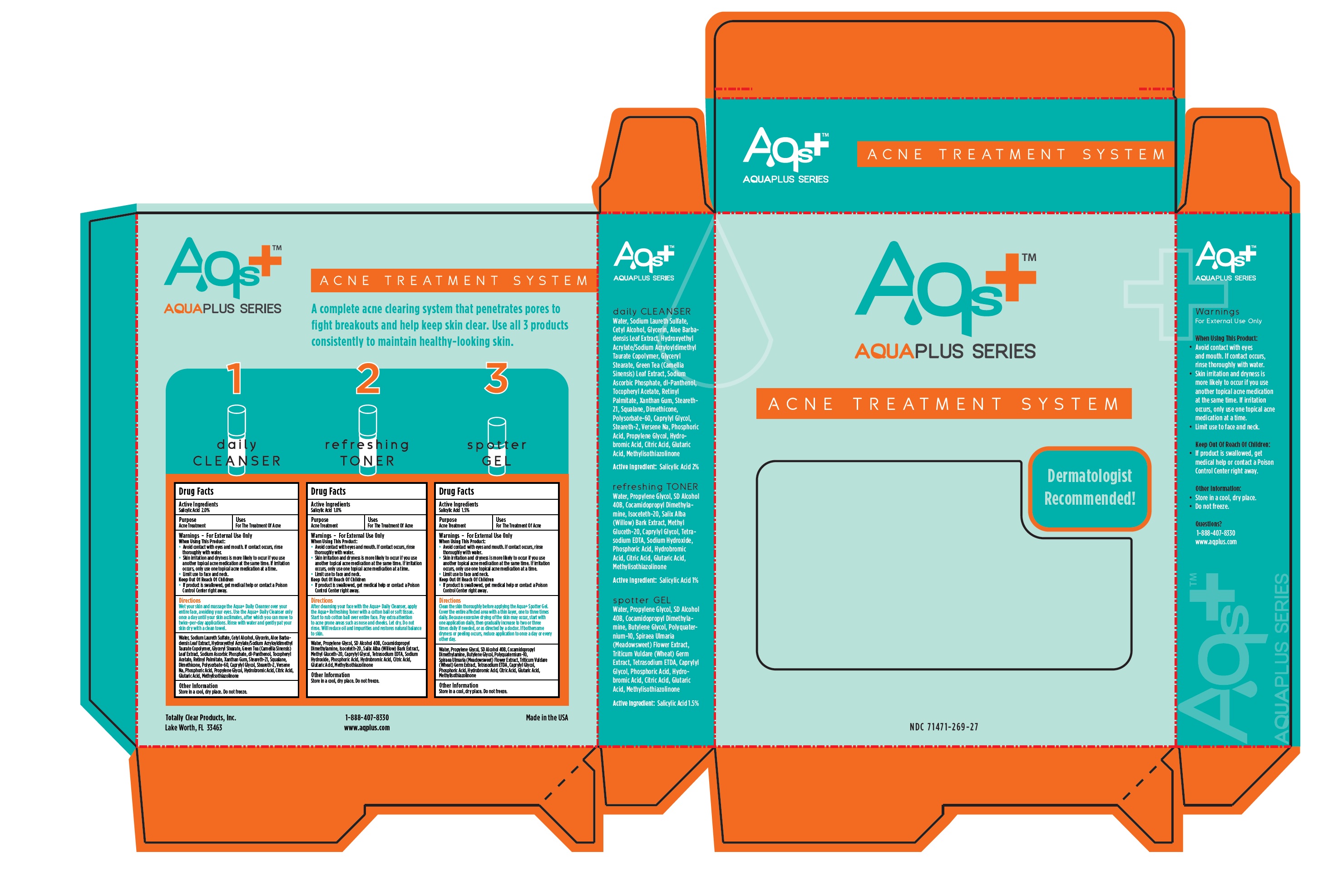
Aqua Plus Acne Treatment System
daily CLEANSER
Drug Facts
Warnings - For External Use Only
When Using This Product:
- Avoid contact with eyes and mouth. If contact occurs, rinse thoroughly with water.
- Skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time.
- Limit use to face and neck.
Water, Sodium Laureth Sulfate, Cetyl Alcohol, Glycerin, Aloe Barbadensis Leaf Extract, Hydroxyethyl Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Glyceryl Stearate, Green Tea (Camellia Sinensis) Leaf Extract, Sodium Ascorbic Phosphate, dl-Panthenol, Tocopheryl Acetate, Retinyl Palmitate, Xanthum Gum, Steareth-21, Squalane, Dimethicone, Polysorbate-60, Cprylyl Glycol, Steareth-2, Versene Na, Phosphoric Acid, Propylene Glycol, Hydrobromic Acid, Citric Acid, Glutaric Acid, Methylisothiazolinone
refreshing TONER
Drug Facts
Warnings - For External Use Only
When Using This Product:
- Avoid contact with eyes and mouth. If contact occurs, rinse thoroughly with water.
- Skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time.
- Limit use to face and neck.
spotter GEL
Drug Facts
Warnings - For External Use Only
When Using This Product:
- Avoid contact with eyes and mouth. If contact occurs, rinse thoroughly with water.
- Skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time.
- Limits use to face and neck.
Directions
Clean the skin thoroughly before applying the Aqua+ Spotter Gel. Cover the entire affected area with a thin layer, one to three times daily. Because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed, or as directed by a doctor. If bothersome dryness or peeling occurs, reduce application to once a day or every other day.
Water, Propylene Glycol, SD Alcohol 40B, Cocamidopropyl Dimethylamine, Butylene Glycol, Polyquaternium-10, Spirea ulmaria (Meadowsweet) Flower Extract, Triticum Vuldare (Wheat) Germ Extract, Tetrasodium ETDA, Caprylyl Glycol, Phosphoric Acid, Hydrobromic Acid, Citric Acid, Glutaric Acid, Methylisothiazolinone