INDICATIONS AND USAGE
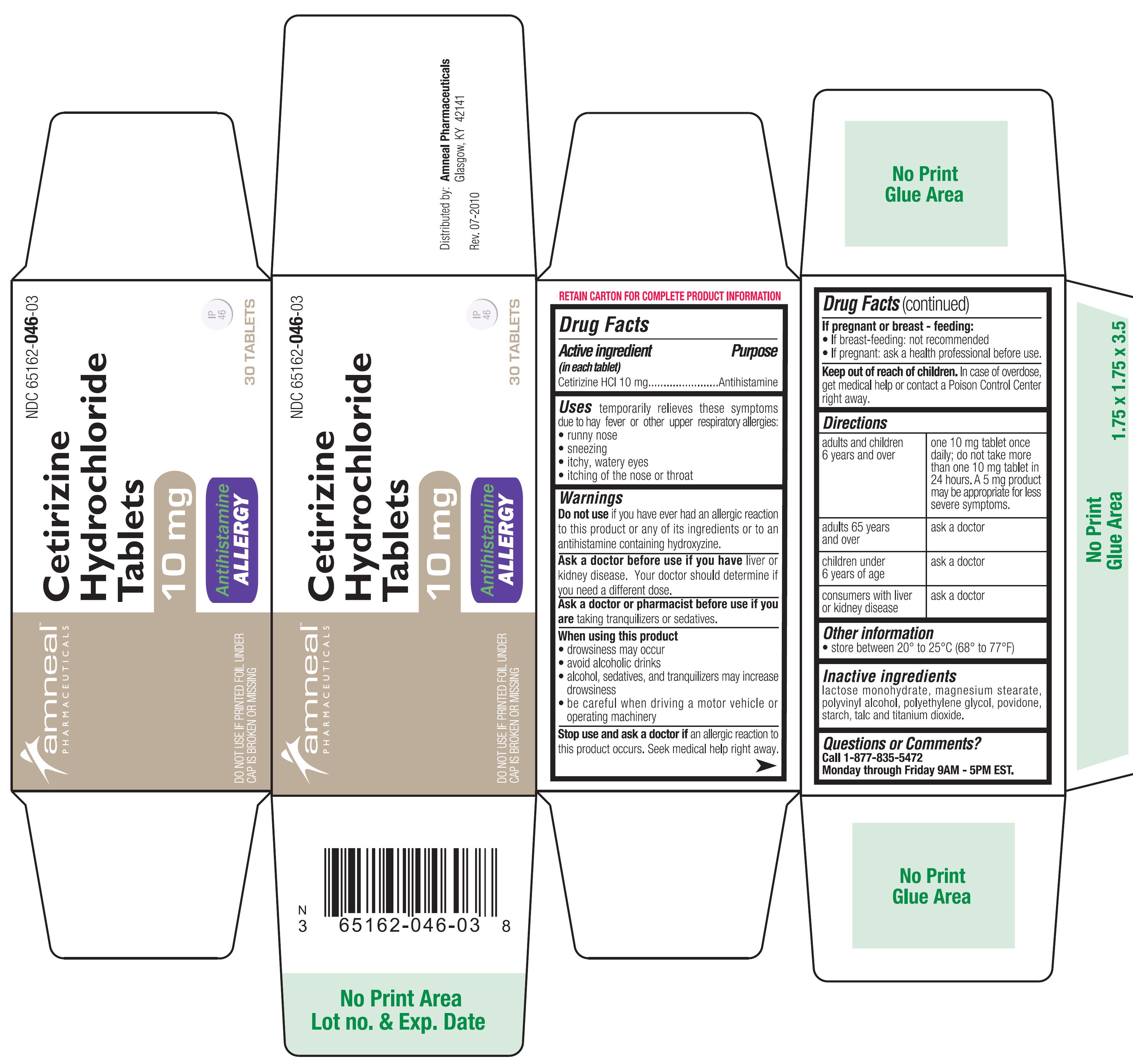
temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
● runny nose
● sneezing
● itchy, watery eyes
● itching of the nose or throat
DO NOT USE
Do not use if you have ever had an allergic reaction to this product or any of its ingredients or to an antihistamine containing hydroxyzine.
ASK DOCTOR
Ask a doctor before use if you have liver or kidney disease. Your doctor should determine if you need a different dose.
ASK DOCTOR/PHARMACIST
Ask a doctor or pharmacist before use if you are taking tranquilizers or sedatives.
When using this product
● drowsiness may occur
● avoid alcoholic drink
● alcohol, sedatives, and tranquilizers may increase drowsiness
● be careful when driving a motor vehicle or operating machinery
WARNINGS
Stop use and ask a doctor if an allergic reaction to this product occurs. Seek medical help right away.
PREGNANCY OR BREAST FEEDING
If pregnant or breast-feeding :
• if breast-feeding: not recommended
• if pregnant: ask a health professional
before use.
KEEP OUT OF REACH OF CHILDREN
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
DOSAGE AND ADMINISTRATION
| Adults andchildren 6years andover | One 10 mg tablet once daily; do not take more than one 10 mg tablet in 24 hours. A 5 mg product may be appropriate for less sever symptoms. |
| Adults 65years andover | Ask a doctor. |
| Childrenunder 6 yearsof age | ask a doctor |
| Consumerswith liver orkidneydisease | ask a doctor |