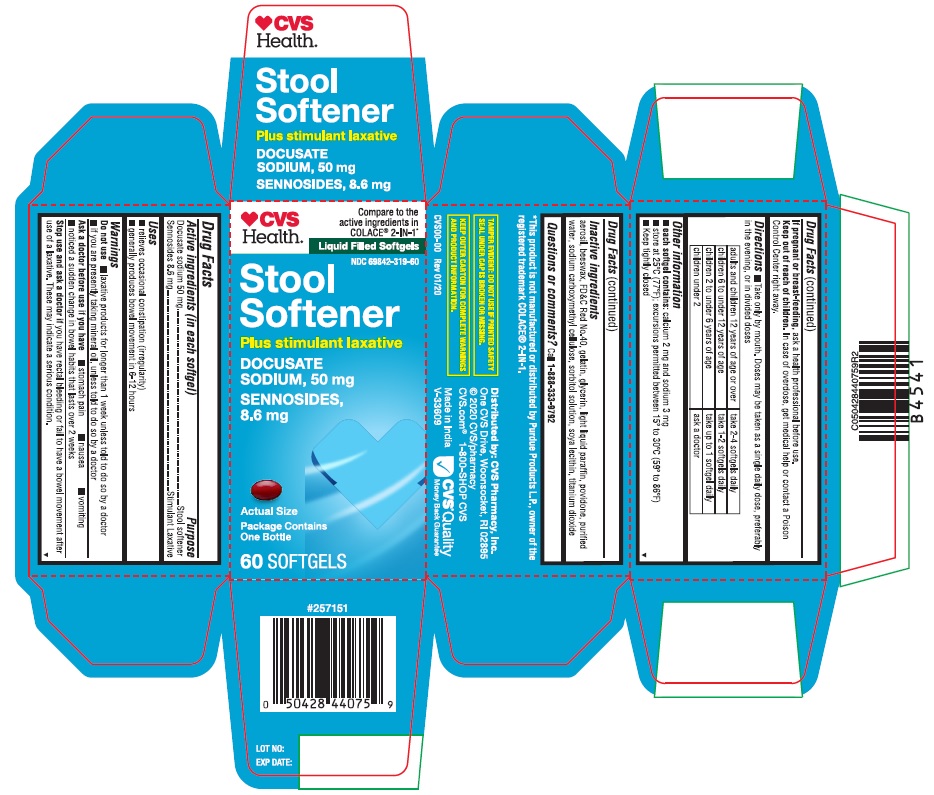
| Active ingredients (in each softgel) | Purpose |
| Docusate sodium 50 mg | Stool softener |
| Sennosides 8.6 mg | Stimulant Laxative |
Uses
- relieves occasional constipation (irregularity)
- generally produces bowel movement in 6-12 hours
Warnings
Do not use
- laxative products for longer than 1 week unless told to do so by a doctor
- if you are presently taking mineral oil, unless told to do so by a doctor
Ask a doctor before use if you have
- stomach pain
- nausea
- vomiting
- noticed a sudden change in bowel habits that lasts over 2 weeks
Directions
- Take only by mouth. Doses may be taken as a single daily dose, preferably in the evening, or in divided doses
| adults and children 12 years of age or over | take 2-4 softgels daily |
| children 6 to under 12 years of age | take 1-2 softgels daily |
| children 2 to under 6 years of age | take upto 1 softgel daily |
| children under 2 | ask a doctor |
Other information
- each softgel contains: calcium 2 mg and sodium 3 mg
- store at 25°C (77°F); excursions permitted between 15°-30°C (59°-86°F)
- Keep tightly closed
Inactive ingredients
Aerosil, beeswax, FD&C Red No.40, gelatin, glycerin, light liquid paraffin, povidone (PVP K-30), purified water, sodium carboxymethylcellulose, sorbitol solution, soya lecithin, titanium dioxide
PRINCIPAL DISPLAY PANEL
♥CVSHealth™
Compare to the
active ingredients in
COLACE® 2-IN-1*
Liquid Filled Softgels
NDC 69842-319-60
Stool Softener
DOCUSATE SODIUM, 50 mg
SENNOSIDES, 8.6 mg
Plus stimulant laxative
Package Contains One Bottle
60 SOFTGELS
Actual Size
*This product is not manufactured or distributed by Purdue Products L.P., owner of the
registered trademark COLACE® 2-IN-1.