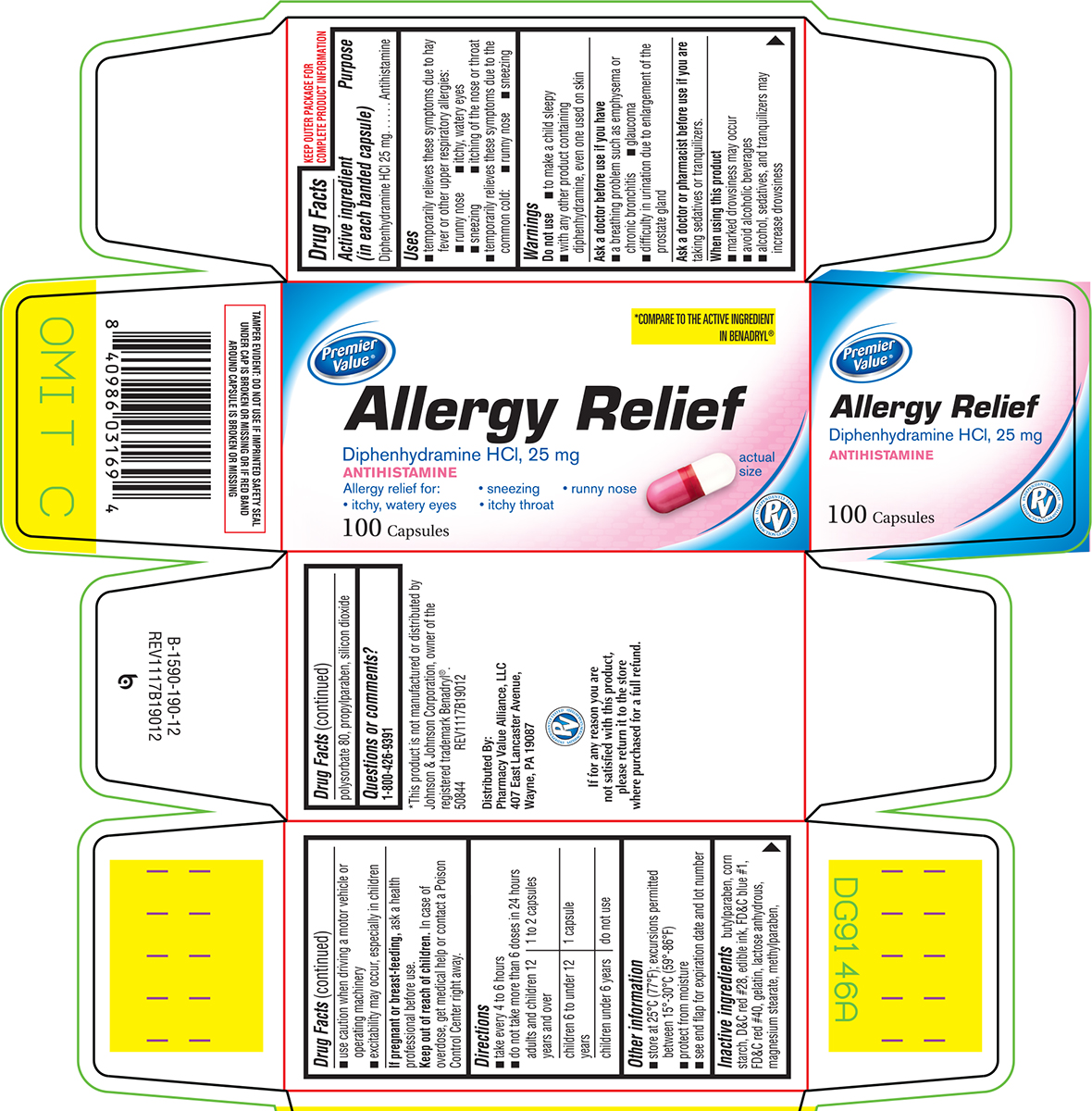
Uses
- temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- itchy, watery eyes
- sneezing
- itching of the nose or throat
- temporarily relieves these symptoms due to the common cold:
- runny nose
- sneezing
Warnings
Do not use
- to make a child sleepy
- with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- difficulty in urination due to enlargement of the prostate gland
Directions
- take every 4 to 6 hours
- do not take more than 6 doses in 24 hours
| adults and children 12 years and over | 1 to 2 capsules |
| children 6 to under 12 years | 1 capsule |
| children under 6 years | do not use |
Other information
- store at 25°C (77°F); excursions permitted between 15°-30°C (59°-86°F)
- protect from moisture
- see end flap for expiration date and lot number
Inactive ingredients
butylparaben, corn starch, D&C red #28, edible ink, FD&C blue #1, FD&C red #40, gelatin, lactose anhydrous, magnesium stearate, methylparaben, polysorbate 80, propylparaben, silicon dioxide
Principal Display Panel
Premier
Value®
*COMPARE TO THE ACTIVE INGREDIENT
IN BENADRYL®
Allergy Relief
Diphenhydramine HCl, 25 mg
ANTIHISTAMINE
Allergy relief for:
• sneezing
• runny nose
• itchy, watery eyes
• itchy throat
actual size
100 Capsules
PV
INDEPENDENTLY TESTED
SATISFACTION GUARANTEED
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY SEAL
UNDER CAP IS BROKEN OR MISSING OR IF RED BAND
AROUND CAPSULE IS BROKEN OR MISSING
*This product is not manufactured or distributed
by Johnson & Johnson Corporation, owner of the
registered trademark Benadryl®.
50844 REV1117B19012
Distributed By:
Pharmacy Value Alliance, LLC
407 East Lancaster Avenue,
Wayne, PA 19087
If for any reason you are
not satisfied with this product,
please return it to the store
where purchased for a full refund.
Premier Value 44-190