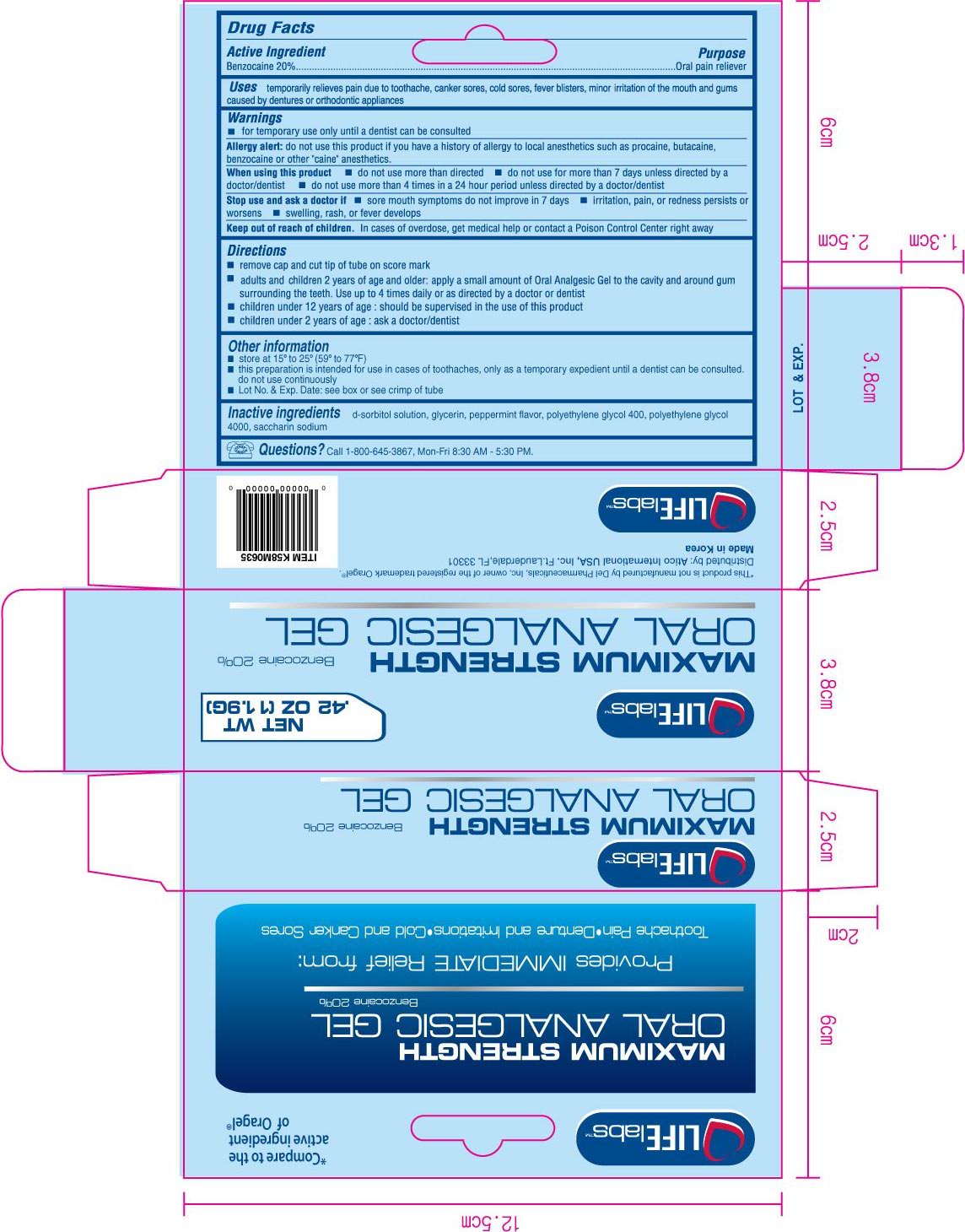
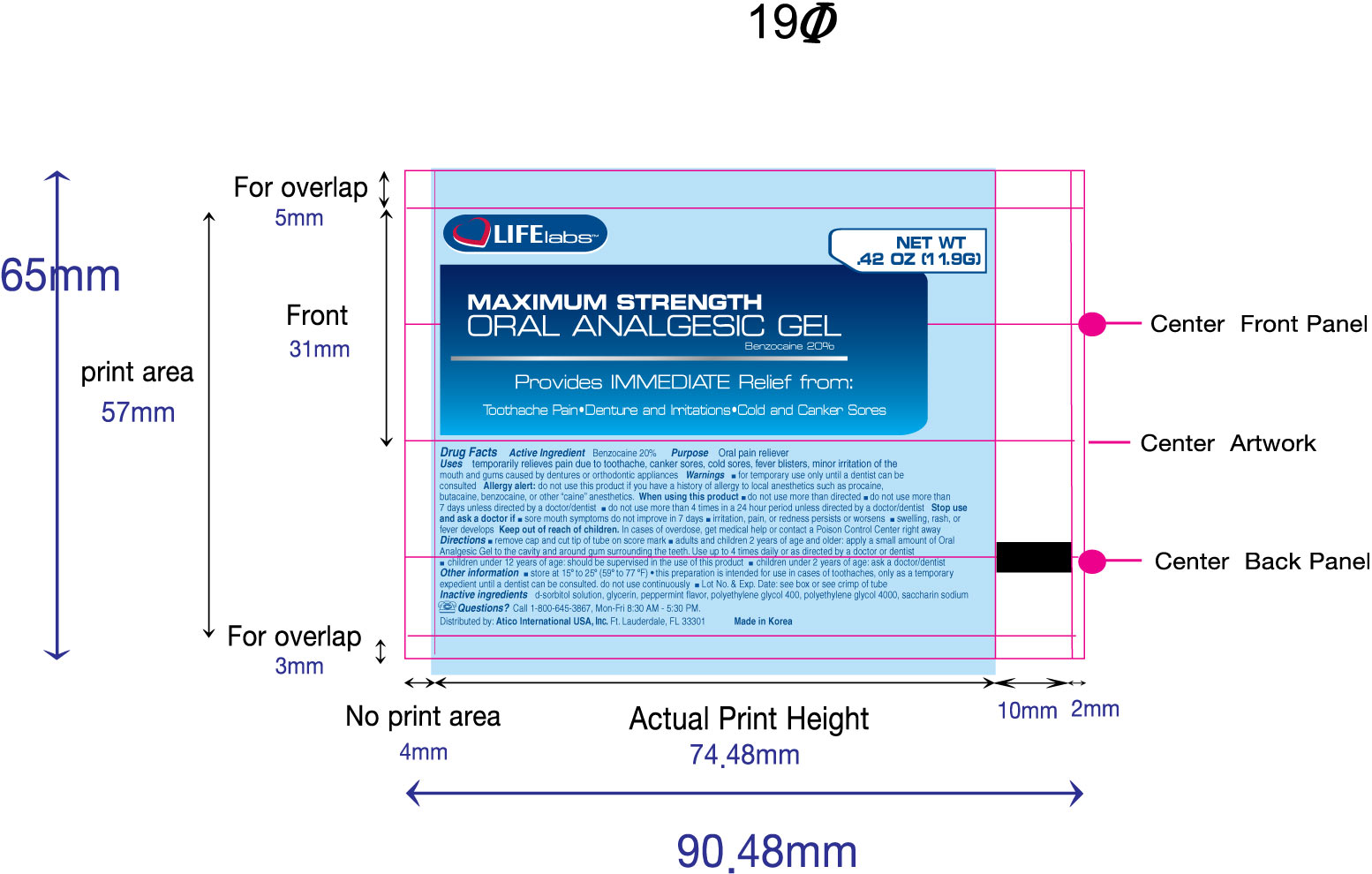
Purpose
Oral pain reliever
Uses
temporarily relieves pain due to toothache, canker sores, cold sores, fever blisters, minor irritation of the mouth and gums caused by dentures or orthodontic appliances
Warnings
for temporary use only until a dentist can be consulted
Allergy alert:
do not use this product if you have a history of allergy to local anesthetics such a procaine, butacaine, benzocaine or other "caine" anesthetics.
When using this product
- do not use more than directed
- do not use for more than 7 days unless directed by a doctor/dentist
- do not use more than 4 times in a 24 hour period unless directed by a doctor/dentist
Stop use and ask a doctor if
- sore mouth symptoms do not improve in 7 days
- irritation, pain, or redness persists or worsens
- swelling, rash, or fever develops
Keep out of reach of children.
In cases of overdose, get medical help or contact a Poison Control Center right away.
Directions
- remove cap and cut tip of tube on score mark
- adults and children 2 years of age and older: apply a small amount of Oral Analgesic Gel to the cavity and around gum surrounding the teeth. use up to 4 times daily or as directed by a doctor or dentist.
- children under 12 year of age: should be supervised in the use of this product
- children under 2 years of age: ask a doctor/dentist
Other information
- store at 15o to 25o (50o to 77oF)
- This preparation is intended for use in cases of toothaches, only as a temporary expedient until a dentist can be consulted. Do not use continuously
- Lot No. and Exp. Date: see box or see crimp of tube