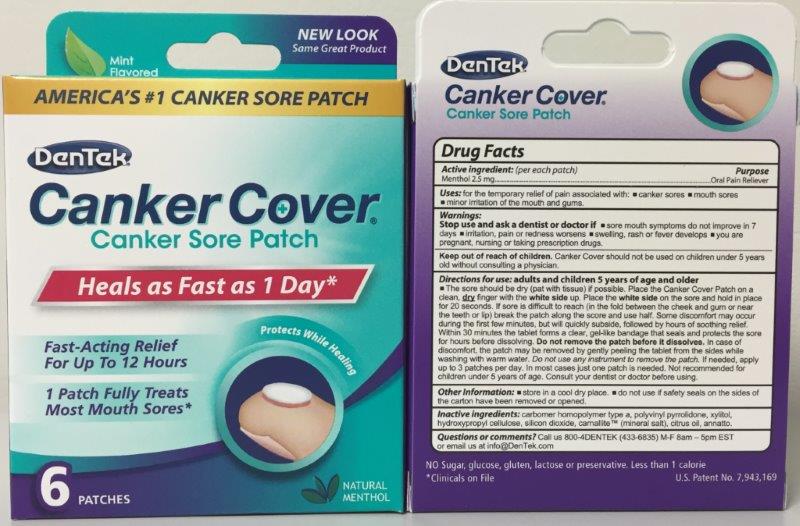
DENTEK CANKER COVER- menthol patch, extended release
Bershtel Enterprises
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active Ingredient
menthol: 2.5 mg
Keep Out of Reach of Children Section
Warning: Keep out of reach of children.
Purpose Section
For Relief of pain associated with: canker sores & mouth sores.
Ask Doctor
Consult your dentist or doctor before using.
Indications & Usage
Adult and Children 5 year of age and older.
The sore should be dry (pat with tissue) is possible. Place the Canker Cover Patch on a clean, dry finger with the white side up. Place the white side on the sore and hold in place for 20 seconds. Within 30 minutes the tablet forms a clear, gel-like bandage that seals and protects the sore for hours before disolving. DO NOT REMOVE PATCH BEFORE IT DISSOLVES.
Dosage & Administration
2.5 mg per patch
Questions section
Call us 800-4Dentek (433-6835) M-F 8am - 5am EST or email us at Info@Dentek.com
Warning Section
Keep out of reach of children. If irritation, pain, or redness worsens, if swelling, rash or fever develops, or if sore mouth systoms persists for 7 days, see your doctor or dentist.
Inactive Ingredients
- citrus medica fruit
- povidone
- colloidal silicon dioxide
- carbomer 934
- Xylitol
- Hydroxypropyl
- sea salt
- magnesium stearate