IBUPROFEN- ibuprofen tablet, film coated
GREENBRIER INTERNATIONAL, INC.
----------
Assured 44-291-Delisted
Active ingredient (in each brown tablet)
Ibuprofen USP, 200 mg (NSAID)*
*nonsteroidal anti-inflammatory drug
Uses
- temporarily relieves minor aches and pains due to:
- headache
- toothache
- backache
- menstrual cramps
- the common cold
- muscular aches
- minor pain of arthritis
- temporarily reduces fever
Warnings
Allergy alert: Ibuprofen may cause a severe allergic reaction, especially in people allergic to aspirin. Symptoms may include:
- hives
- shock
- facial swelling
- rash
- blisters
- skin reddening
- asthma (wheezing)
If an allergic reaction occurs, stop use and seek medical help right away.
Stomach bleeding warning: This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you:
- have had stomach ulcers or bleeding problems
- take a blood thinning (anticoagulant) or steroid drug
- are age 60 or older
- take other drugs containing prescription or nonprescription NSAIDs [aspirin, ibuprofen, naproxen, or others]
- have 3 or more alcoholic drinks every day while using this product
- take more or for a longer time than directed
Heart attack and stroke warning: NSAIDs, except aspirin, increase the risk of heart attack, heart failure, and stroke. These can be fatal. The risk is higher if you use more than directed or for longer than directed.
Do not use
- if you have ever had an allergic reaction to any other pain reliever/fever reducer
- right before or after heart surgery
Ask a doctor before use if
- stomach bleeding warning applies to you
- you have a history of stomach problems, such as heartburn
- you have high blood pressure, heart disease, liver cirrhosis, kidney disease, asthma, or had a stroke
- you are taking a diuretic
- you have problems or serious side effects from taking pain relievers or fever reducers
Ask a doctor or pharmacist before use if you are
- under a doctor's care for any serious condition
- taking aspirin for heart attack or stroke, because ibuprofen may decrease this benefit of aspirin
- taking any other drug
Stop use and ask a doctor if
- you experience any of the following signs of stomach bleeding:
- feel faint
- vomit blood
- have bloody or black stools
- have stomach pain that does not get better
- you have symptoms of heart problems or stroke
- chest pain
- slurred speech
- leg swelling
- trouble breathing
- weakness in one part or side of body
- pain gets worse or lasts more than 10 days
- fever gets worse or lasts more than 3 days
- redness or swelling is present in the painful area
- any new symptoms appear
Directions
- do not take more than directed
- the smallest effective dose should be used
- adults and children 12 years and over: take 1 tablet every 4 to 6 hours while symptoms persist
- if pain or fever does not respond to 1 tablet, 2 tablets may be used
- do not exceed 6 tablets in 24 hours, unless directed by a doctor
- children under 12 years: ask a doctor
Other information
- store between 20º-25ºC (68º-77ºF)
- avoid excessive heat 40ºC (104ºF)
- see end flap for expiration date and lot number
Inactive ingredients
carnauba wax, colloidal silicon dioxide, corn starch, hypromellose, lactose anhydrous, magnesium stearate, microcrystalline cellulose, polydextrose, polyethylene glycol, red iron oxide, sodium starch glycolate, stearic acid, titanium dioxide
Principal Display Panel
ASSURED™
COMPARE TO ACTIVE INGREDIENT OF
ADVIL® TABLETS†
Ibuprofen
• Ibuprofen Tablets USP, 200 mg
Pain reliever / Fever reducer (NSAID)
Actual Size
100 tablets
TAMPER EVIDENT: DO NOT USE IF IMPRINTED
SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
†This product is not manufactured or distributed by
Wyeth Consumer Healthcare, owner of the registered
trademark Advil® Tablets.
50844 REV1116B29112
DISTRIBUTED BY
GREENBRIER INTERNATIONAL, INC.
500 VOLVO PARKWAY, CHESAPEAKE, VA 23320
Assured 44-291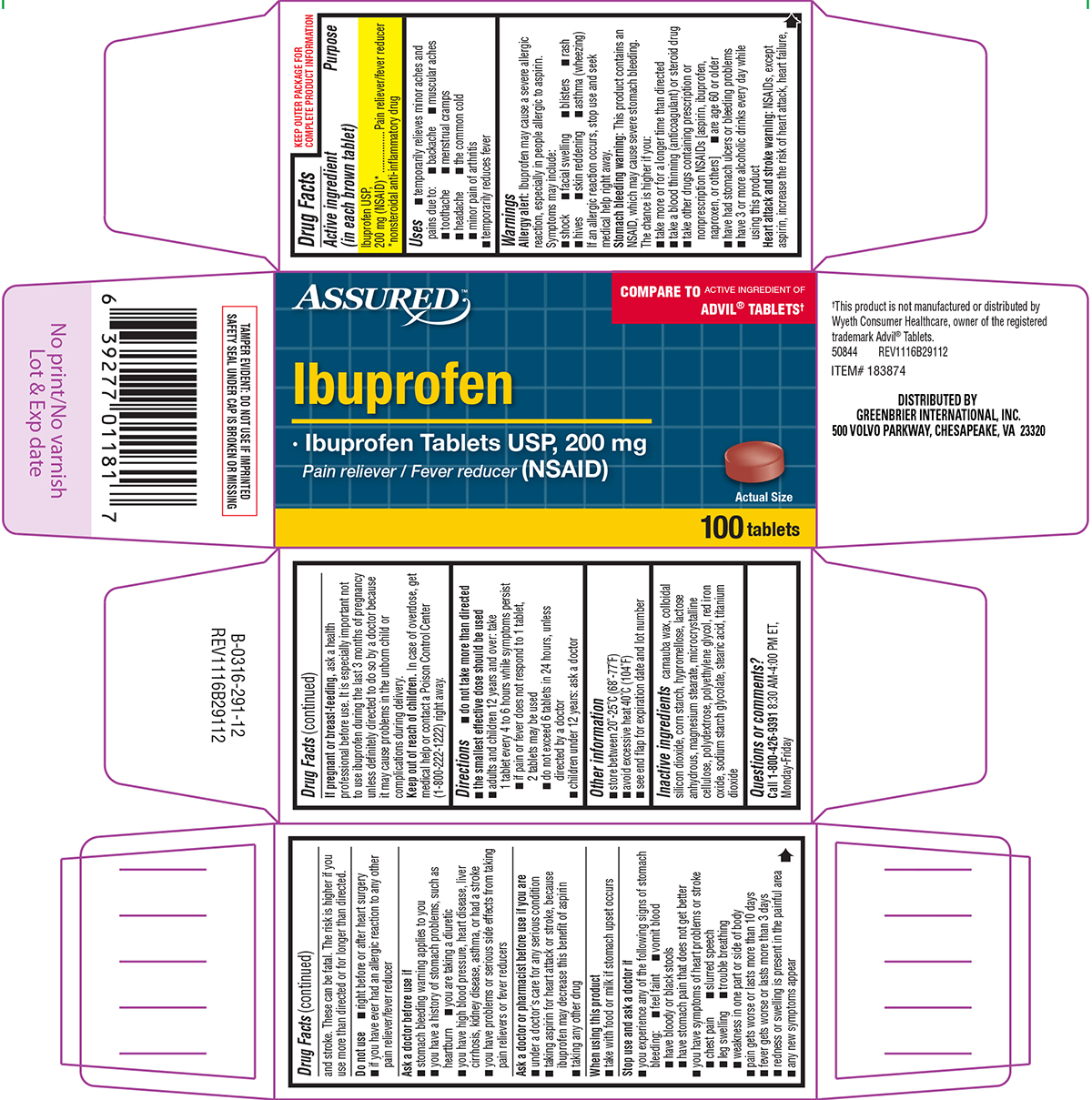
| IBUPROFEN
ibuprofen tablet, film coated |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - GREENBRIER INTERNATIONAL, INC. (610322518) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 038154464 | pack(33992-0291) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 832867894 | manufacture(33992-0291) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 832867837 | pack(33992-0291) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 967626305 | pack(33992-0291) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 868734088 | pack(33992-0291) | |