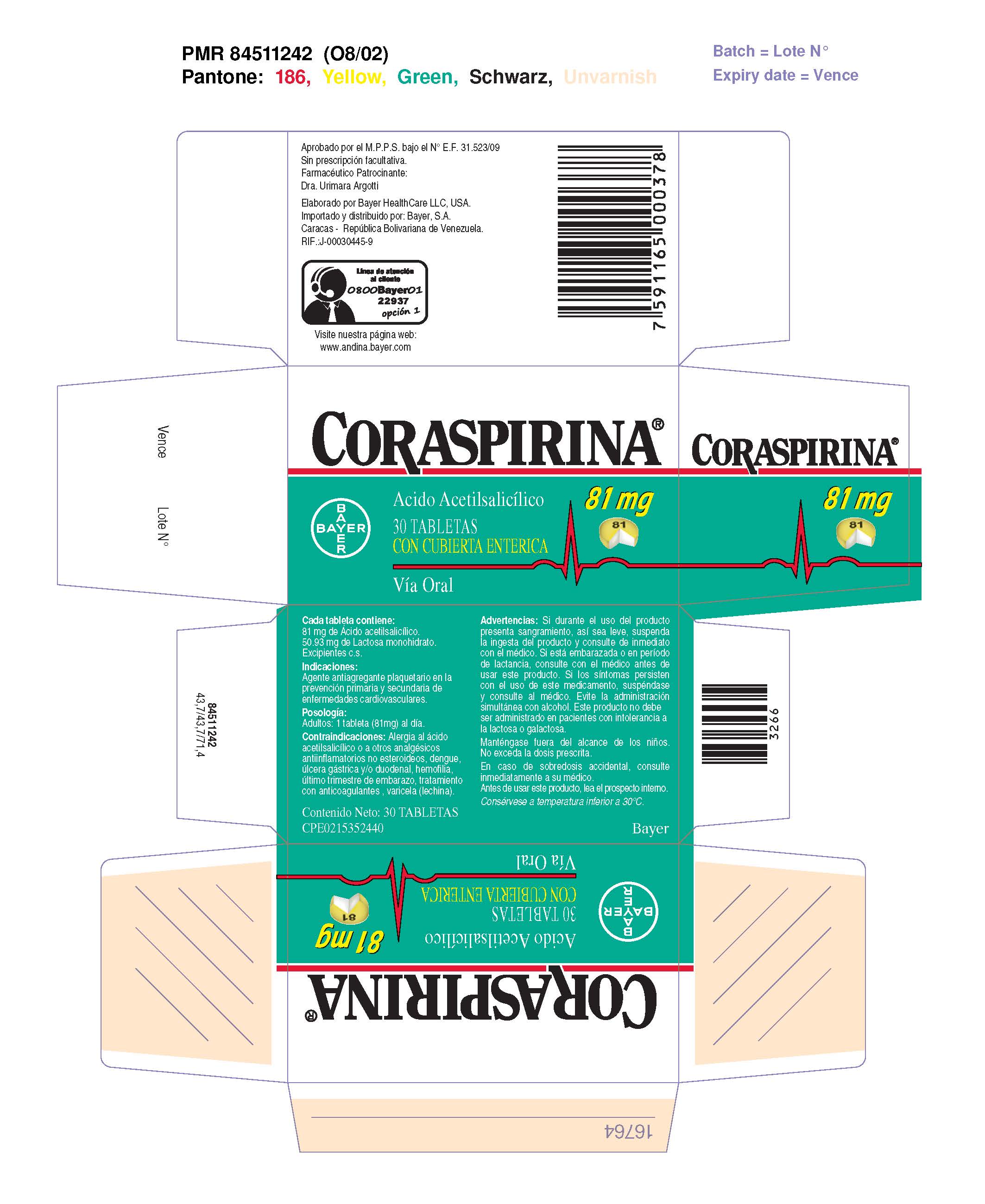
CORASPIRINA 81 MG TABLETAS CON CUBIERTA ENTERICA- coraspirin 81 mg enteric coasted tablet tablet, coated
Bayer HealthCare LLC.
----------
| CORASPIRINA 81 MG TABLETAS CON CUBIERTA ENTERICA
coraspirin 81 mg enteric coasted tablet tablet, coated |
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| Labeler - Bayer HealthCare LLC. (112117283) |