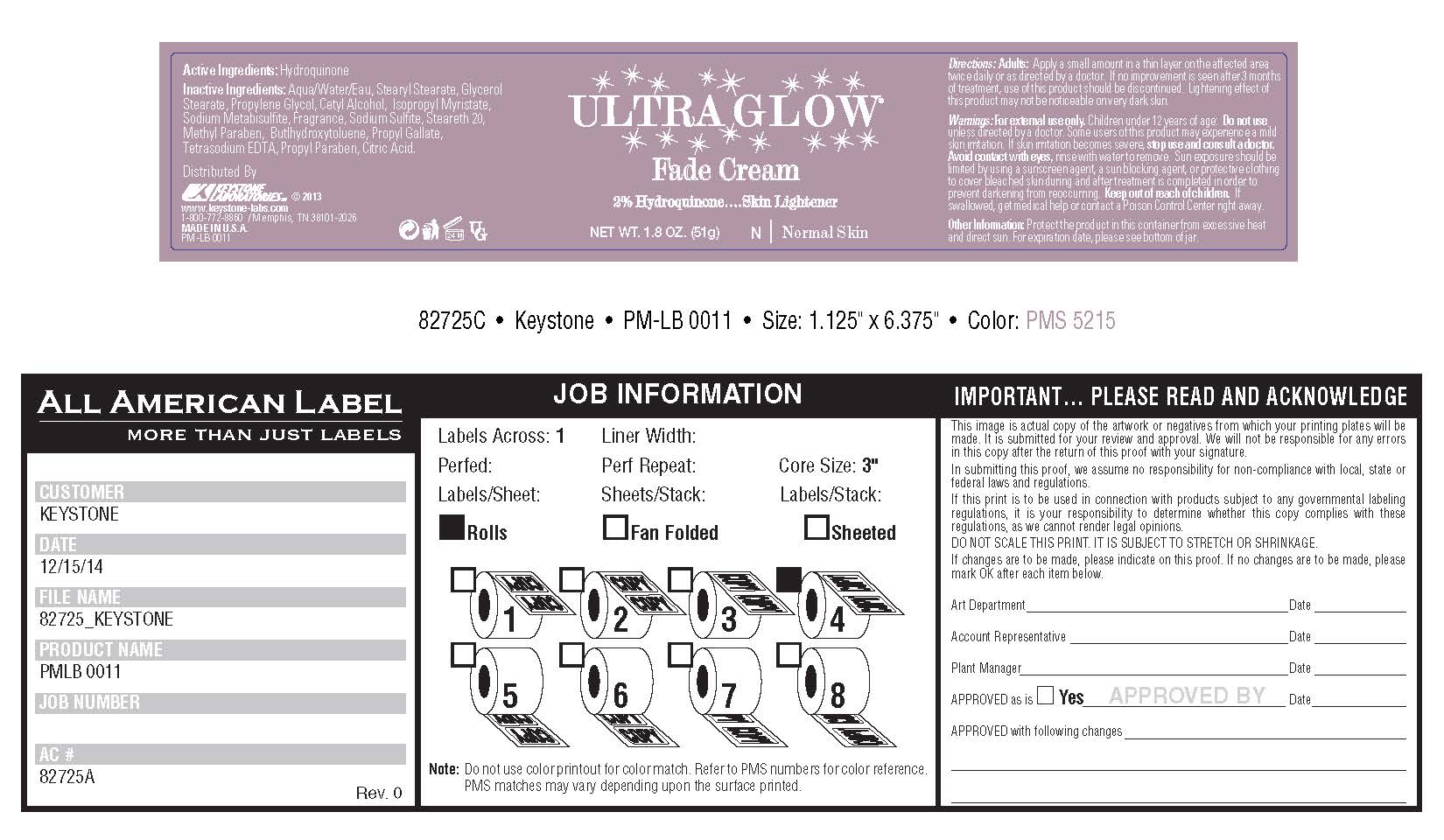
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Warnings:
For external use only. Children under 12 years of age: Do not use unless directed by a doctor. Some users of this product may experience a mild skin irritation. If skin irritation becomes severe, stop use and consult a doctor. Avoid contact with eyes, rinse with water to remove. Sun exposure should be limited by using a sunscreen agent, a sun blocking agent, or protective clothing to cover bleached skin during and after treatment is completed in order to prevent darkening from reoccurring. Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions:
Adults: Apply a small amount in a thin layer on the affected area twice daily or as directed by a doctor. If no improvement is seen after 3 months of treatment, use of this product should be discontinued. Lightening effect of this product may not be noticeable on very dark skin.
Other Information: Protect the product in this container from excessive heat and direct sun. For expiration date, please see bottom of jar.
Distributed by
KEYSTONE LABORATORIES © 2013
www.keystone-labs.com
1-800-772-8860 / Memphis, TN 38101-2026
MADE IN U.S.A.
PM-LB 0011