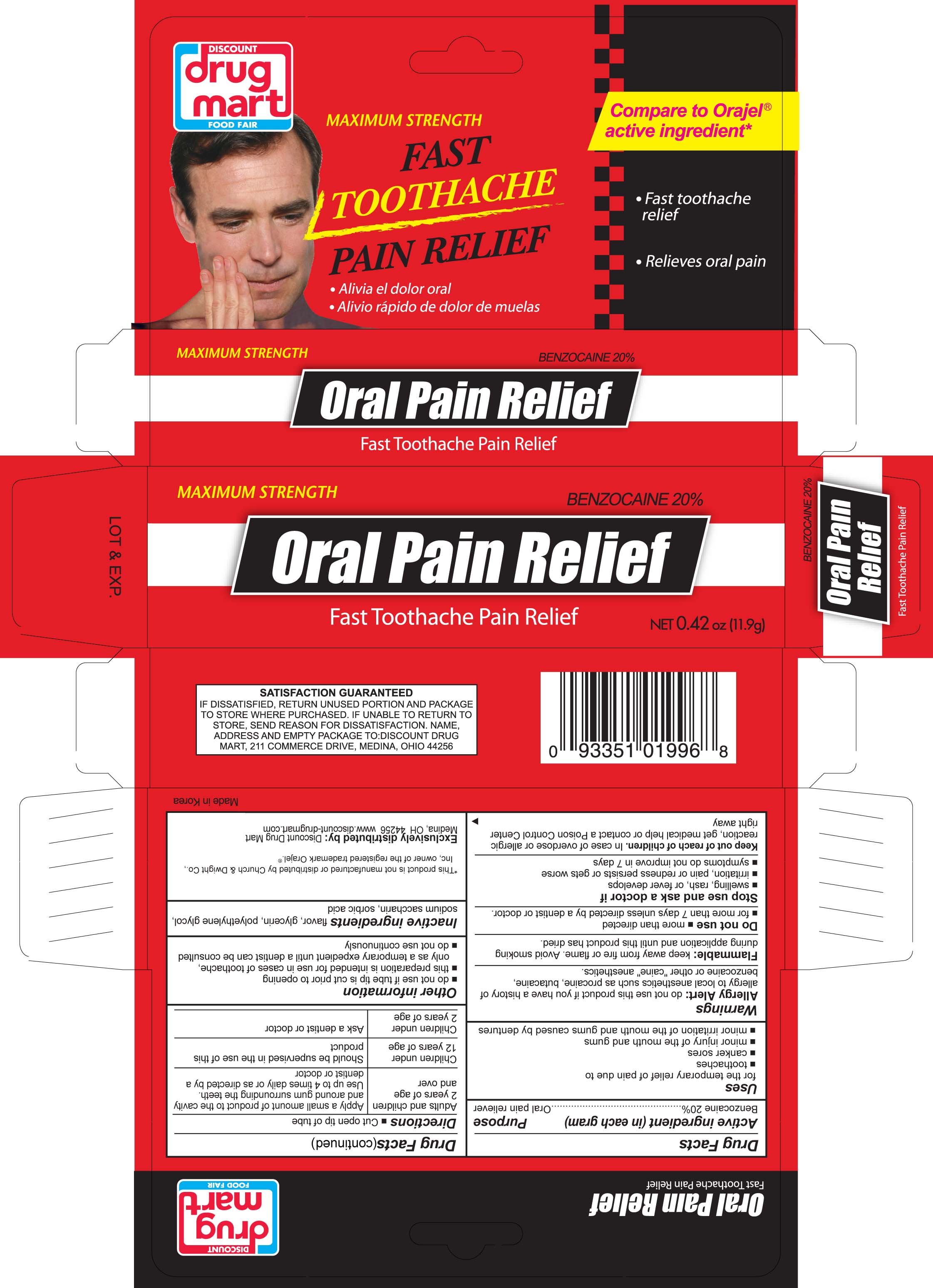
ORAL PAIN RELIEF- oral pain reliever gel
Discount Drug Mart, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient (in each gram)
Benzocaine 20%
Purpose
Oral Pain Reliever
Uses
for the temporary relief of pain due to
- toothaches
- canker sores
- minor injury of the mouth and gums
- minor irritation of the mouth and gums caused by dentures
Warnings
Allergy alert: do not use this product if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine or other "caine" anesthetics
Flammable: keep away from fire or flame. Avoid smoking during application and until this product has dried.
Do not use
more than directed for more than 7 days unless told to do so by a dentist or doctor
Stop use and ask a doctor if
swelling, rash or fever develops; irritation, pain or redness persists or worsens; symptoms do not improve in 7 days
Keep out of reach of children. In case of overdose or allergic reaction, get medical help or contact a Poison Control Center right away.
Directions
Directions cut open tip of tube
| Adults and children 2 years of age and over | Apply a small amount of product to the cavity and around gum surrounding the teeth. Use up to 4 times daily or as directed by a dentist or doctor |
|
Children under 12 years of age
| Should be supervised in the use of this product |
| Children under 2 years of age | Ask a dentist or doctor |
Inactive ingredients
Inactive ingredients flavor, glycerin, polyethylene glycol, sodium saccharin, sorbic acid,
Other Information
- do not use if tube tip is cut prior to opening
- this preparation is intended for use in case of toothache, only as a temporary expedient until a dentist can be consulted
- do not use continuously
Discount Drug Mart Oral Pain Relief
Discount Drug Mart, Inc.
