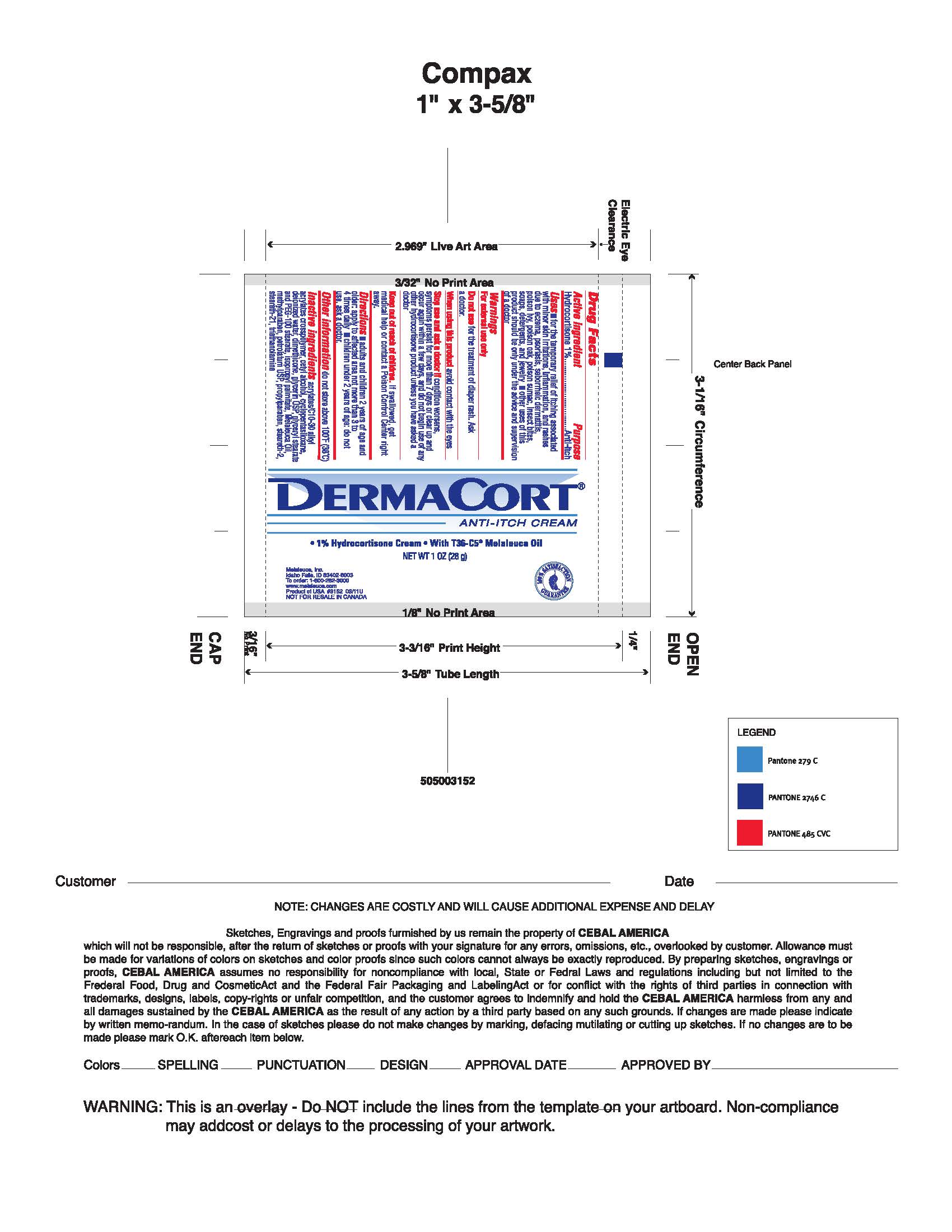
Uses
- For the temporary relief of itching associated with minor skin irritations, inflammation and rashes due to eczema, psoriasis, , seborrheic dermatitis, poison ivy, poison oak, poison sumac, insect bites, soaps, detergents, and jewelery
- Other use of this product should be only under the advice and supervision of a doctor.
Stop use and ask a doctor if conditions worsens, or if symptoms persist for more than 7 days or clear up and occur again within a few days, stop use of this product and do not begin use of any other hydrocortisone product unless you have consulted a doctor.
Keep out of reach of children.
In case of accidental ingestion, seek professional assistance or contact a Poison Control Center immediately.
Directions adults and children 2 years of age and older: Apply to affected area not more than 3 to 4 times daily. Children under 2 years of age: Do not use, consult a doctor.
Inactive ingredients Acrylates/C10-30 Alkyl Acrylates Crosspolymer, Cetyl Alcohol, Cyclopentasiloxane, Deionized Water, Dimethicone, Glycerin USP, Glyceryl Stearate and PEG-100 Stearate, Isopropyl Palmitate, Melaleuca Oil, Methylparaben, Petrolatum USP, Propylparaben, Serareth-2, Steareth-21, Triethanolamine.