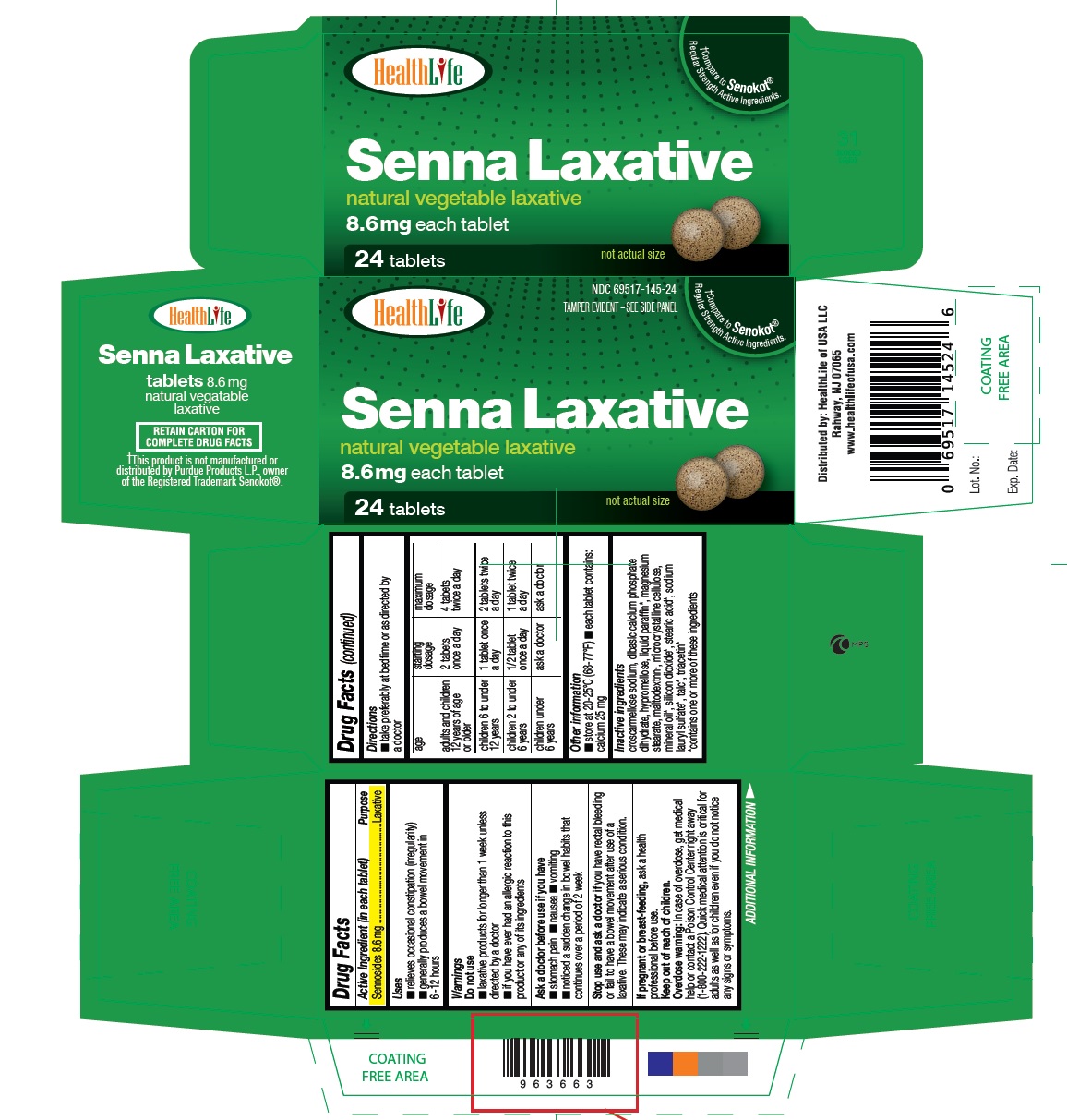
Uses
- relieves occasional constipation (irregularity)
- generally produces a bowel movement in 6-12 hours
Warnings
Do not use
- laxative products for longer than 1 week unless directed by a doctor
- if you have ever had an allergic reaction to this product or any of its ingredients
Ask a doctor before use if you have
- stomach pain
- nausea
- vomiting
- noticed a sudden change in bowel habits that continues over a period of 2 week
Stop use and ask a doctor if
you have rectal bleeding or fail to have a bowel movement after use of a laxative. These may indicate a serious condition.
Overdose warning
In case of overdose, get medical help or contact a Poison Control Center right away (1-800-222-1222). Quick medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
Directions
- take preferably at bedtime or as directed by a doctor
| age | starting dosage | maximum dosage |
| adults and children 12 years of age or older | 2 tablets once a day | 4 tablets twice a day |
| children 6 to under 12 years | 1 tablet once a day | 2 tablets twice a day |
| children 2 to under 6 years | 1/2 tablet once a day | 1 tablet twice a day |
| children under 6 years | ask a doctor | ask a doctor |
Inactive ingredients
croscarmellose sodium, dibasic calcium phosphate dihydrate, hypromellose, liquid paraffin, magnesium stearate, maltodextrin, microcrystalline cellulose, mineral oil*, silicon dioxide*,stearic acid*,sodium lauryl sulfate*,talc*,triacetin*
[*contains one or more of these ingredients]