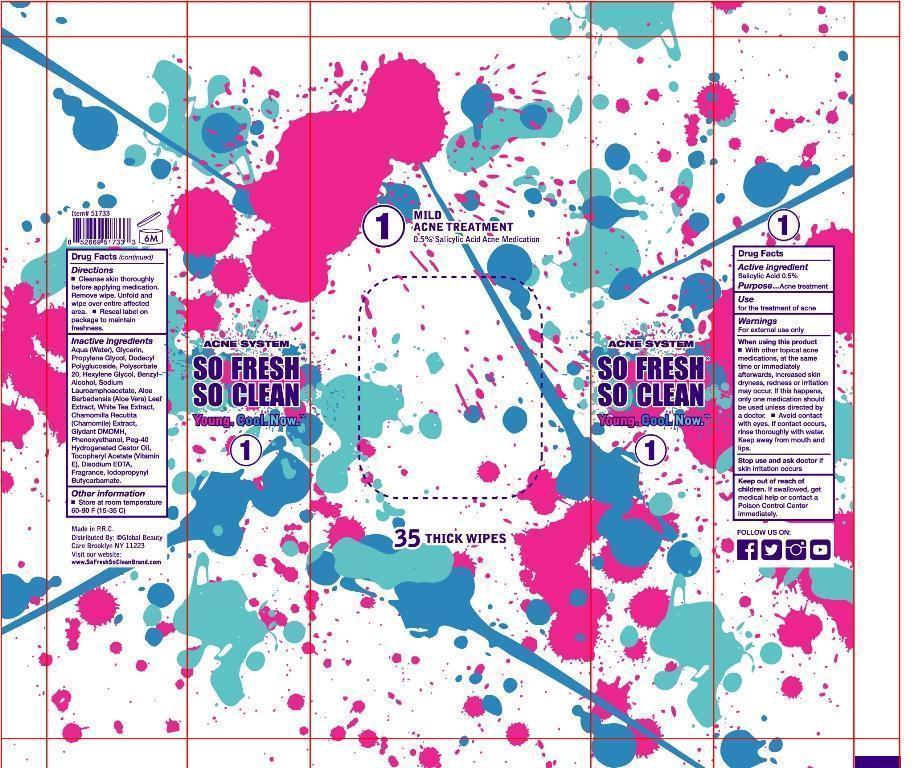
Warnings
for external use only
when using this product
with other topical acne medications ,at the same time or immediately afterwards ,increased skin dryness ,redness or
irritation may occur .If this happens ,only one medication should be used unless directed by a doctor.
avoid contact with eyes .if contact occurs, rinse thoroughly with water. keep away from mouth and lips.
stop use and ask doctor if skin irritation occurs.
Keep out of reach of children ,If swallowed ,get medical help or contact a Poison Control Center immediately.
Directions
cleanse skin thoroughly before applying medication ,remove wipe ,unfold and wipe over entire affected area.
reseal label on package to maintain freshness.
aqua (water),glycerin, propylene glycol , dodecyl polyglucoside, polysorbate 20,HEXYLENE GLYCOL , BENZYL ALCOHOL ,SODIUM LAURAMINOPROPIONATE ,ALOE BARBADENSIS(ALOE VERA )EXTRACT ,WHITE TEA EXTRACT ,CHAMOMILLA RECUTITA(CHAMOMILE)EXTRACT ,GLYDANT DMDM H,PHENOXYETHANOL ,PEG-40 HYDROGENATED CASTOR OIL,TOCOPHERYL ACETATE(VITAMIN E) ,DISODIUM EDTA,IODOPROPYNYL BUTYLCARBAMATE