CLEARASIL RAPID RESCUE DEEP TREATMENT PADS- salicylic acid cloth
RB Health (US) LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
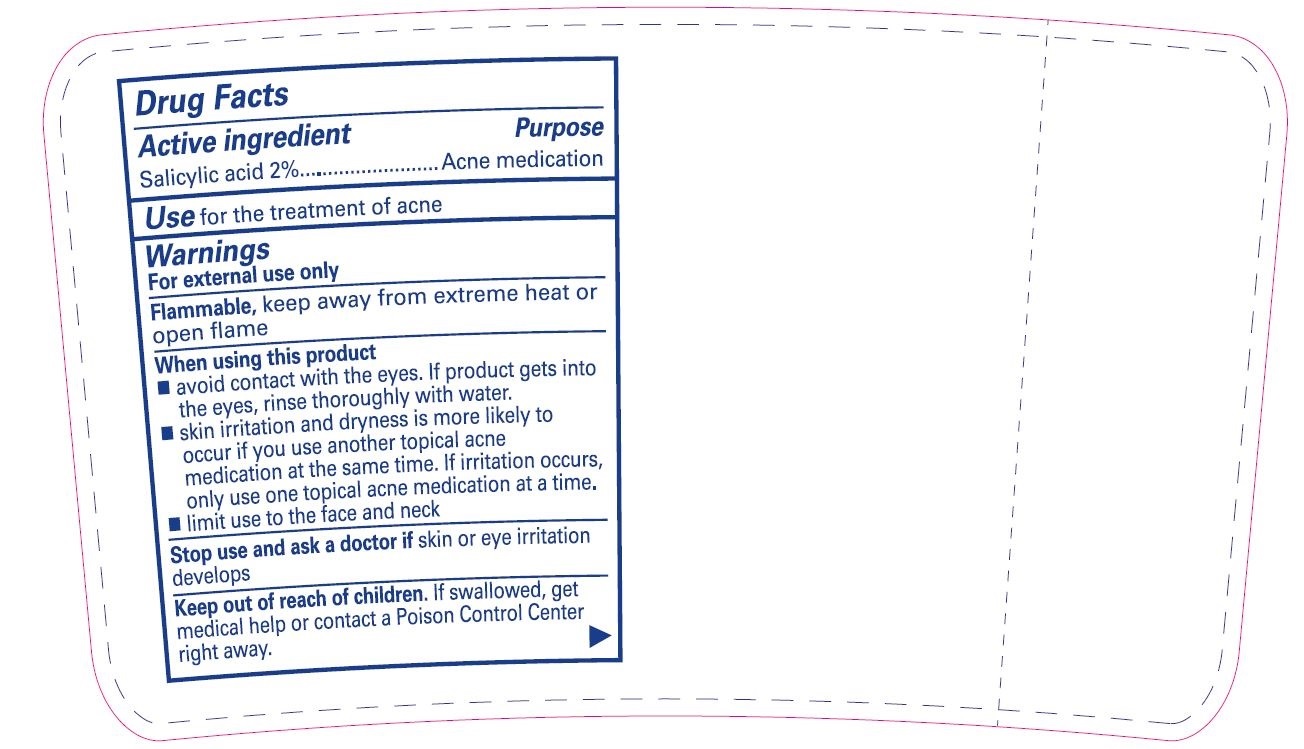
Active ingredient
Salicylic acid 2%
Use
for the treatment of acne
Warnings
For external use only
Flammable, keep away from extreme heat or open flame
When using this product
- avoid contact with the eyes. If product gets into the eyes, rinse thoroughly with water.
- skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time.
- limit use to the face and neck
Stop use and ask a doctor if skin or eye irritation develops
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
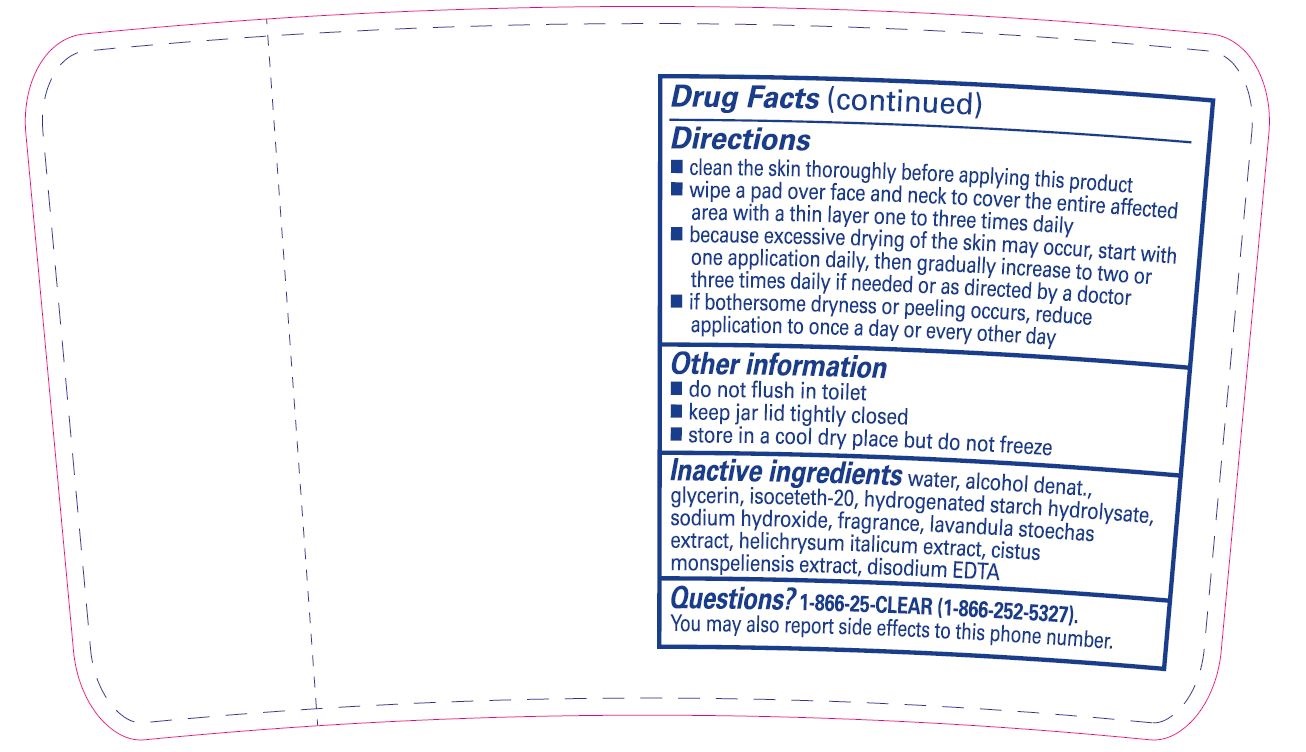
Directions
- clean the skin thoroughly before applying this product
- wipe a pad over face and neck to cover the entire affected area with a thin layer one to three times daily
- because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor
- if bothersome dryness or peeling occurs, reduce application to once a day or every other day
Other information
- do not flush in toilet
- keep jar lid tightly closed
- store in a cool dry place but do not freeze
Inactive ingredients
Water, Alcohol Denat., Glycerin, Isoceteth-20, Hydrogenated Starch Hydrolysate, Sodium Hydroxide, Fragrance, Lavandula Stoechas Extract, Helichrysum Italicum Extract, Cistus Monspeliensis Extract, Disodium EDTA
Questions?
1-866-25-CLEAR (1-866-252-5327).
You may also report side effects to this phone number.
Distributed by: RB Health (US),
Parsippany, NJ 07054-0224
Made in France
PRINCIPAL DISPLAY PANEL - 90 Pad Jar Label
Clearasil
®
RAPID RESCUE
DEEP TREATMENT PADS
Salicylic Acid Acne Medication
Maximum Strength
VISIBLE
RESULTS
AS FAST AS
4 HOURS
STARTS TO WORK
INSTANTLY
90 Pads
2.19 in
(5.56 cm) DIA
080917