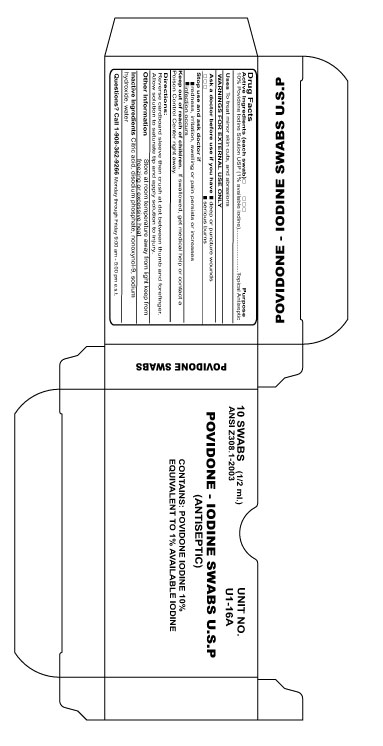
Active Ingredient
10% Povidone Iodine Solution USP (1% available iodine)
Purpose
Topical Antiseptic
Directions
Reverse cardboard sleeve then crush at dot between thumb and forefinger. Allow solution to saturate tip and apply soltion to injury.
Ask a Doctor before use if you have
Deep or puncture wounds
Serious Burns
Stop Use
redness, irritation, swelling or pain persists or increases
infection occurs
Keep Out Of Reach Of Children
If swallowed, get medical help or contact a Poison Control Center right away
Directions
Reverse cardboard sleeve then crush at dot between thumb and forefinger. Allow solution to saturate tip and apply solution to injury.
Other Information
Store at room temperature away from light keep from freezing or excessive heat
Inactive Ingredient
Citric acid, disodium phosphate, nonoxynol-9, sodium hydroxide, water