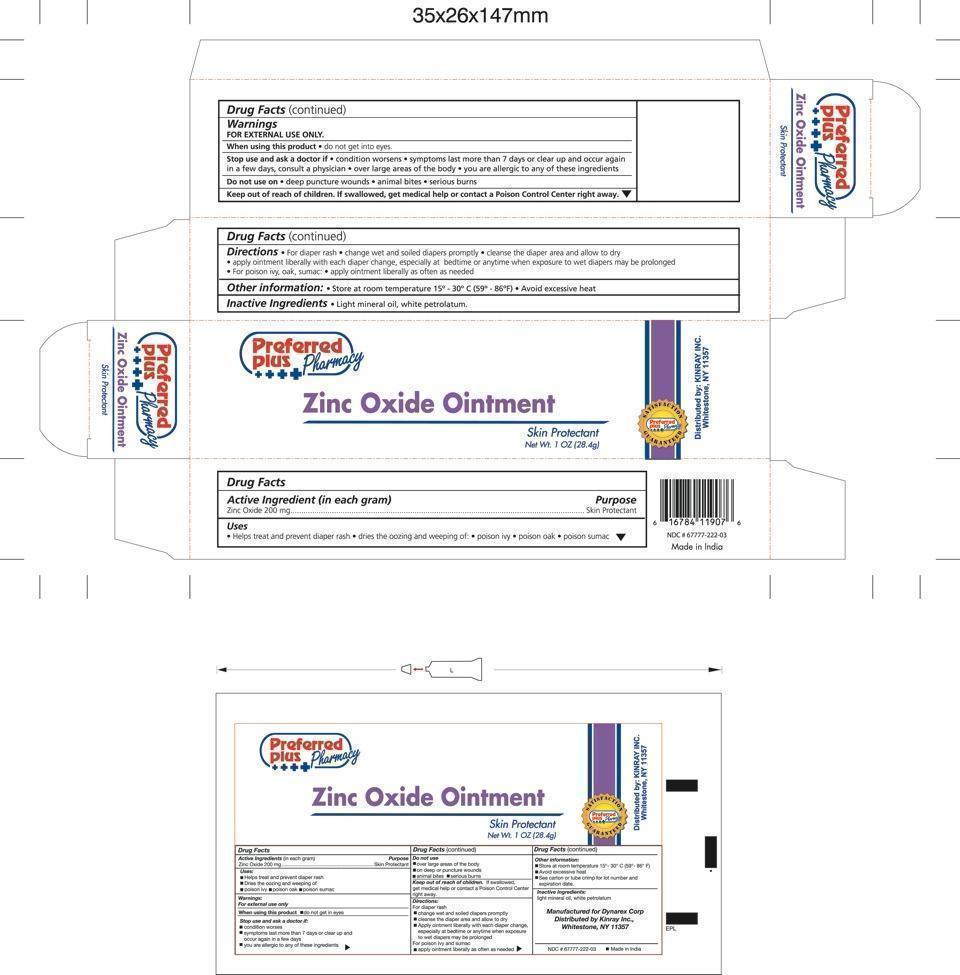
Purpose
Helps treat and prevent diaper rash.
Dries the oozing and weeping of;
poison ivy
poison oak
poison sumac
Stop use and ask a doctor if:
- conditions worsens
- symptoms last more than 7 days or clear up and occur again in a few days, consult a physician.
- over large areas of the body
- if you are allergic to any of these ingredients
Keep out of reach of children
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Dosage and Administration
For diaper rash:
- change wet and soiled diapers promptly
- cleanse the diaper area and allow to dry
- apply ointment liberally with each diaper change, especially at bedtime or anytime when exposure to wet diapers may be prolonged
For poison ivy, poison oak, poison sumac:
- apply liberally as often as needed