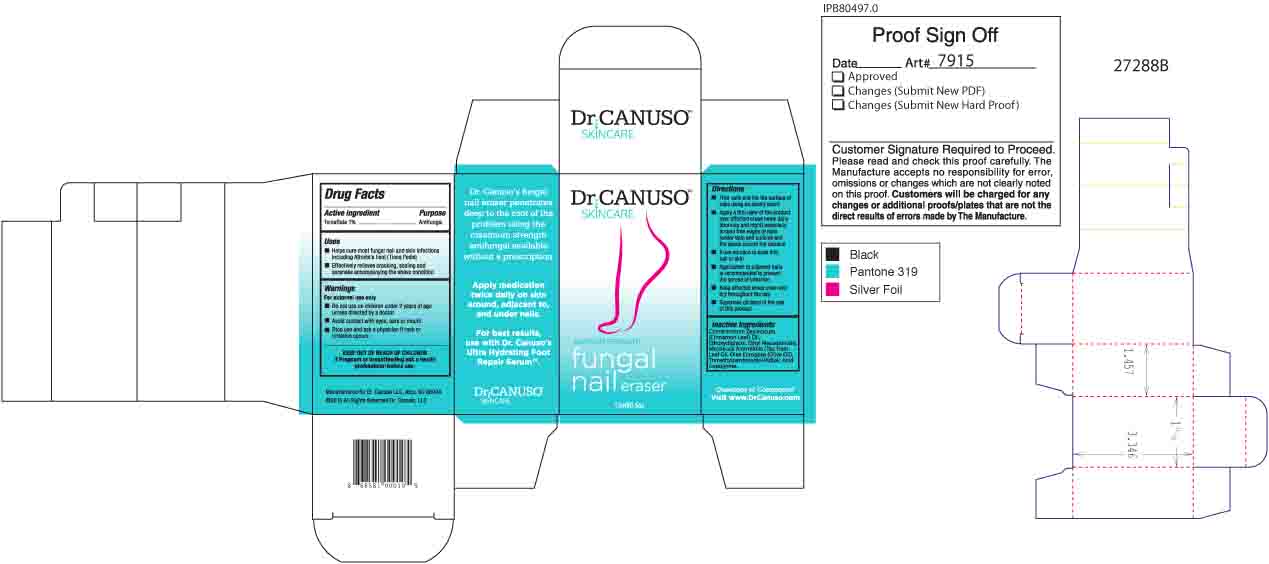
Uses
- Helps cure most fungal nail and skin infections including Fungus (Tinea Pedis)
- Effectively relieves cracking, scaling and soreness accompanying the above conditon
Warnings
For external use only
- Do not use on children under 2 years of age unless directed by a doctor
- Avoid contact with eyes, ears or mouth
- Stop use and ask a physician if rash or irritation occurs
Directions
- Trim nails and file the surface of nails using an emory board
- Apply a thin layer of the product over affected areas twice daily (morning and night) especially around free edges of nails (under tips) and cuticles and the space around the toenails.
- Allow solution to soak into nail or skin
- Application to adjacent nails is recommended to prevent the spread of infection
- Keep affected areas clean and dry throughout the day
- Supervise children in the use of this product
Inactive Ingredients
Cinnamomum Zeylanicum (Cinnamon Leaf) Oil, Ethoxydiglycol, Ethyl Macadamiate, Melaleuca Alternifolia (Tea Tree) Leaf Oil, Olea Europaea (Olive Oil), Trimethylpentanediol/Adipic Acid Copolymer.