Active ingredients
Each drop contains:
Grindelia Aerial Parts 6X
Pilocarpus Leaf 6X
Salvia officinalis (Garden sage) Leaf 6X
Senega officinalis Root 6X
Stramonium (Thorn apple) Aerial Parts 6X
Valeriana officinalis (Valerian) Root 6X
Warnings
Ask a doctor before use if you have
Persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema.
Cough that is accompanied by excessive phlegm (mucus) or fever.
Stop use and ask a doctor if
Cough persists for more than 1 week, tends to recur, or is accompanied by a fever, rash, or
persistent headache. A persistent cough may be a sign of a serious condition.
Symptoms persist or worsen.
If pregnant or breastfeeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact
a Poison Control Center right away.
Stop use and ask a doctor if
Cough persists for more than 1 week, tends to recur, or is accompanied by a fever, rash, or persistent headache. A persistent
cough may be a sign of a serious condition.
Symptoms persist or worsen.
Directions
Adults and adolescents (12 years and older): Take 5 drops three times daily or as
recommended by your healthcare practitioner.
Children (under 12 years): Take under the direction of your
healthcare practitioner.
Uses
For the temporary relief of symptoms associated with cough.
Directions
Adults and adolescents (12 years and older): Take 5 drops three times daily or as
recommended by your healthcare practitioner.
Children (under 12 years): Take under the direction of your
healthcare practitioner.
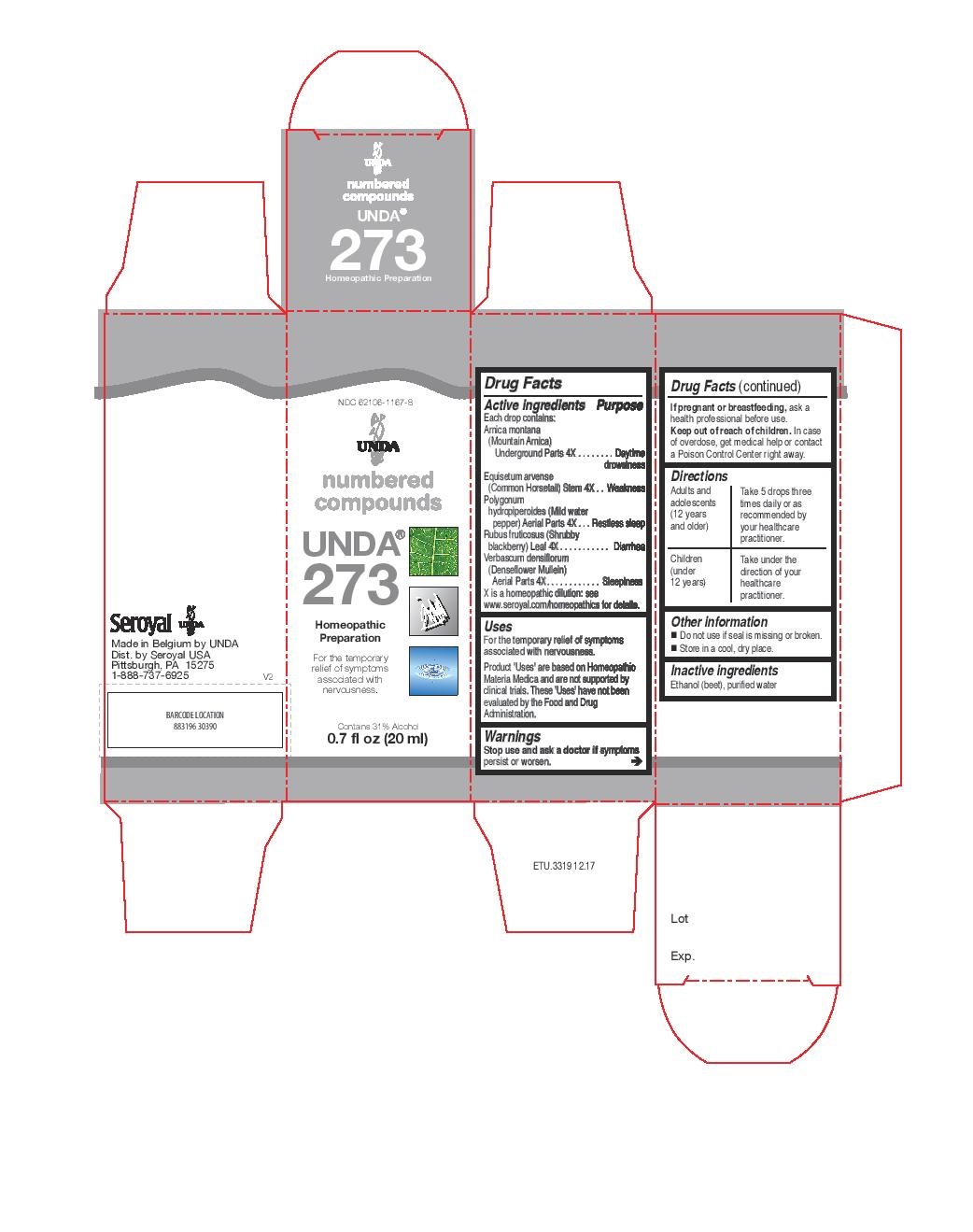
Active ingredients
Each drop contains:
Arnica montana (Mountain Arnica) Root 4X
Equisetum arvense (Common Horsetail) Stem 4X
Polygonum hydropiperoides (Mild water pepper) Whole Plant 4X
Rubus fruticosus (Shrubby blackberry) Leaf 4X
Verbascum densiflorum Aerial Parts 4X
Warnings
Stop use and ask a doctor if symptoms persist or worsen.
If pregnant or breastfeeding, ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
Adults and adolescents (12 years and older): Take 5 drops three times daily or as
recommended by your healthcare practitioner.
Children (under 12 years): Take under the direction of your
healthcare practitioner.
Uses
For the temporary relief of symptoms associated with nervousness.
Directions
Adults and adolescents (12 years and older): Take 5 drops three times daily or as
recommended by your healthcare practitioner.
Children (under 12 years): Take under the direction of your
healthcare practitioner.