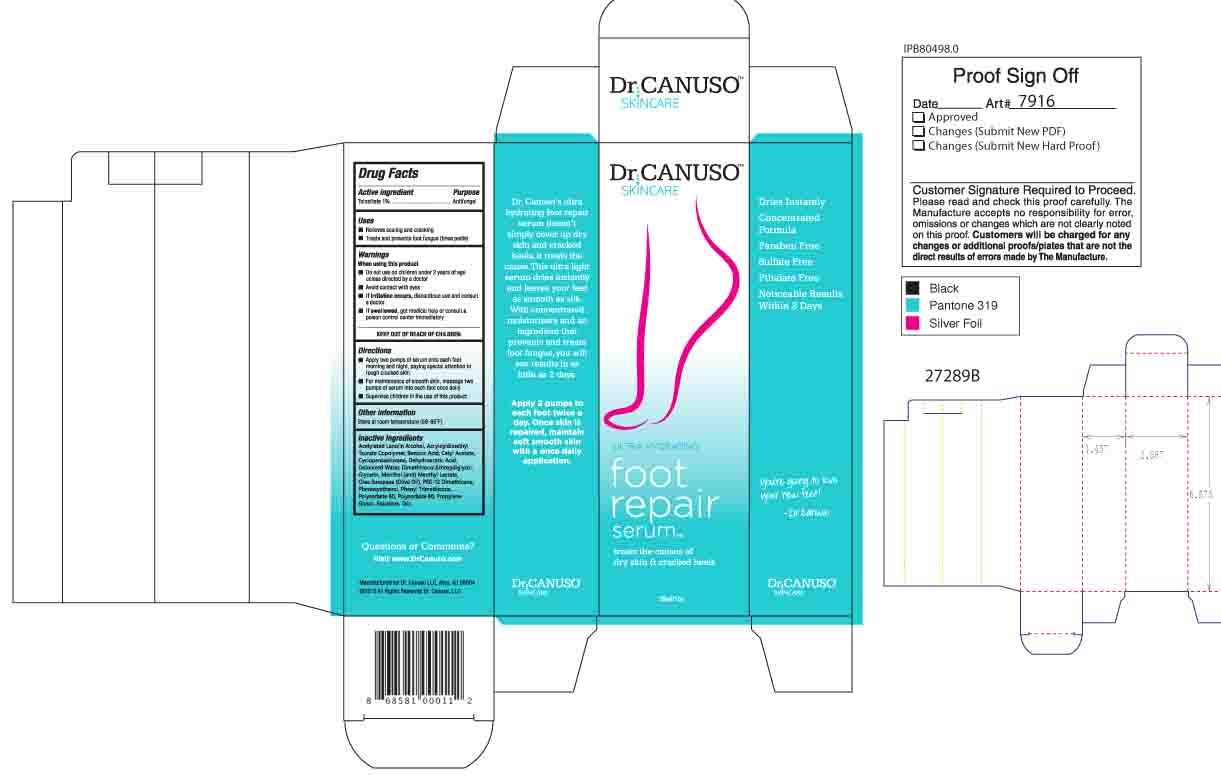
When using this product
- Do not use on children under 2 years of age unless directed by a doctor
- Avoid contact with eyes
- If irritation occurs, discontinue use and consult a doctor
- If swallowed, get medical help or consult a poison control center immediately
Inactive Ingredients
Acetylated Lanolin Alcohol, Acryloyldimethyl Taurate Copolymer, Benzoic Acid, Cetyl Acetate, Cyclopentasiloxane, Dehydroacetic Acid, Deionized Water, Dimethiconol, Ethoxydiglycol, Glycerin, Menthol, Menthyl Lactate, Olea Europaea (Olive Oil), PEG-12 Dimethicone, Phenoxyethanol, Phenyl Trimethicone, Polysorbate 60, Polysorbate 80, Propylene Glycol, Squalane, Talc.
Directions
- Apply two pumps of serum onto each foot morning and night, paying special attention to rough cracked skin.
- For maintenance of smooth skin, massage two pumps of serum into each foot once daily.
- Supervise children in the use of this product.