LACTOVIT ORIGINAL ROLL-ON ANTIPERSPIRANT DEODORANT - aluminum chlorohydrate liquid
Antonio Puig, S.A.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
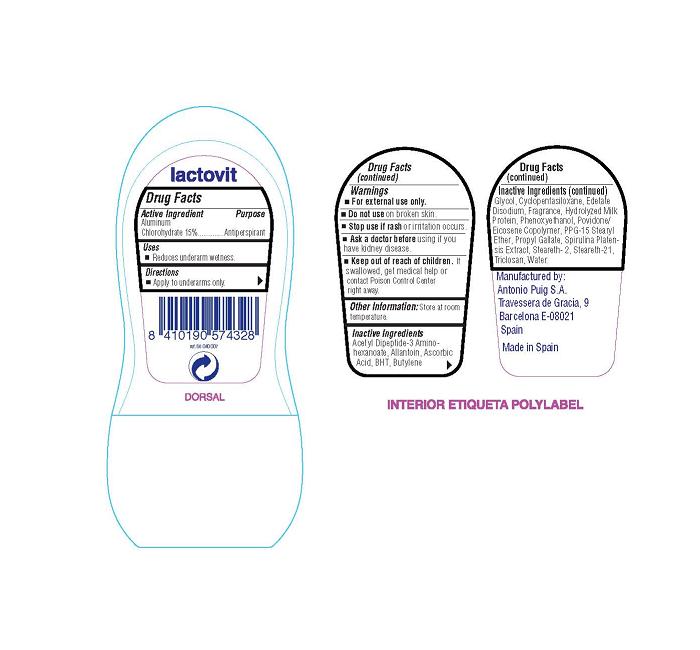
Active Ingredient
Aluminum Chlorohydrate 15 percent
Uses
- Reduces underarm wetness.
Directions
- Apply to underarms only.
- For external use only.
- Do not use on broken skin.
- Stop use if rash or irritation occurs.
- Ask a doctor before using if you have kidney disease.
- Keep out of reach of children. If swallowed, get medical help or
contact Poison Control Center right away.
Other Information:
Store at room temperature.
Inactive Ingredients
Acetyl Dipeptide-3 Amino-hexanoate, Allantoin, Ascorbic Acid, BHT,
Butylene Glycol, Cyclopentasiloxane, Edetate Disodium, Fragrance,
Hydrolyzed Milk Protein, Phenoxyethanol, Povidone/Eicosene
Copolymer, PPG-15 Steryl Ether, Propyl Gallate, Spirulina
Platensis Extract, Steareth-2, Steareth-21, Triclosan, Water
Manufactured by:
Antonio Puig S.A.
Travessera de Gracia, 9
Barcelona E-08021
Spain
Made in Spain
Antonio Puig, S.A.