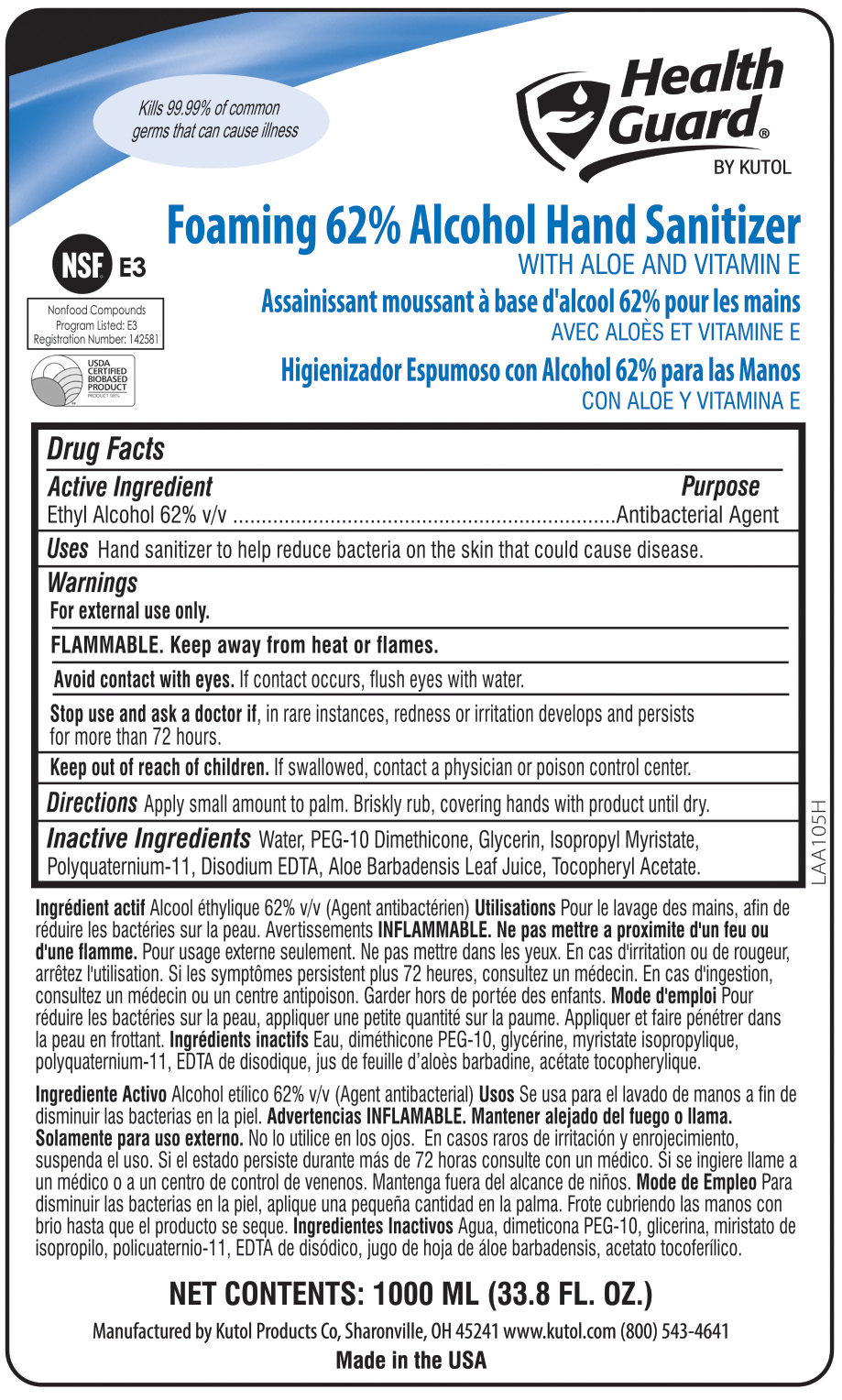
Hand Sanitizer to help reduce bacteria on the skin that could cause disease. Recommended for repeated use.
For external use only.
FLAMMABLE. Keep away from heat or flames.
Avoid contact with eyes. If eye contact occurs, flush with water.
Stop use if, in rare instances, redness or irritation develop. If condition persists for more than 72 hours, consult a physician.
Keep out of reach of children. If swallowed, contact a physician or poison control center.
To decrease bacteria on skin, apply small amount to palm. Briskly rub, covering hands with product until dry.
Hand sanitizer to help reduce bacteria on the skin that could cause disease. Recommended for repeated use.
Avoid contact with eyes. If eye contact occurs, flush with water.
Stop use if, in rare instances, redness or irritation develop. If condition persists for more than 72 hours, consult a physician.
Keep out of reach of children. If swallowed, contact a physician or poison control center.