Uses
- for relief of occasional constipation and irregularity
- this product generally produces bowel movement in 6 to 12 hours
Warnings
Ask a doctor before use if you have
- stomach pain, nausea or vomiting
- a sudden change in bowel habits that lasts more than 2 weeks
When using this product
- do not chew or crush tablet(s)
- do not use within 1 hour after taking an antacid or milk
- you may have stomach discomfort, faintness and cramps
Directions
- take with a glass of water
| adults and children 12 years and over | take 1 to 3 tablets in a single daily dose |
| children 6 to under 12 years | take 1 tablet in a single daily dose |
| children under 6 years | ask a doctor |
Other information
- TAMPER EVIDENT: DO NOT USE IF OUTER PACKAGE IS OPENED OR BLISTER IS TORN OR BROKEN
- avoid excessive humidity
- store at 25°C (77°F); excursions permitted between 15°-30°C (59°-86°F)
- see end flap for expiration date and lot number
Inactive ingredients
acacia, ammonium hydroxide, calcium carbonate, carnauba wax, colloidal anhydrous silica, corn starch, D&C yellow #10 aluminum lake, FD&C yellow #6 aluminum lake, hypromellose, iron oxide black, lactose anhydrous, magnesium stearate, methylparaben, polydextrose, polyethylene glycol, polyvinyl acetate phthalate, povidone, propylene glycol, propylparaben, shellac glaze, simethicone, sodium alginate, sodium benzoate, sodium bicarbonate, stearic acid, sucrose, talc, titanium dioxide, triacetin, triethyl citrate
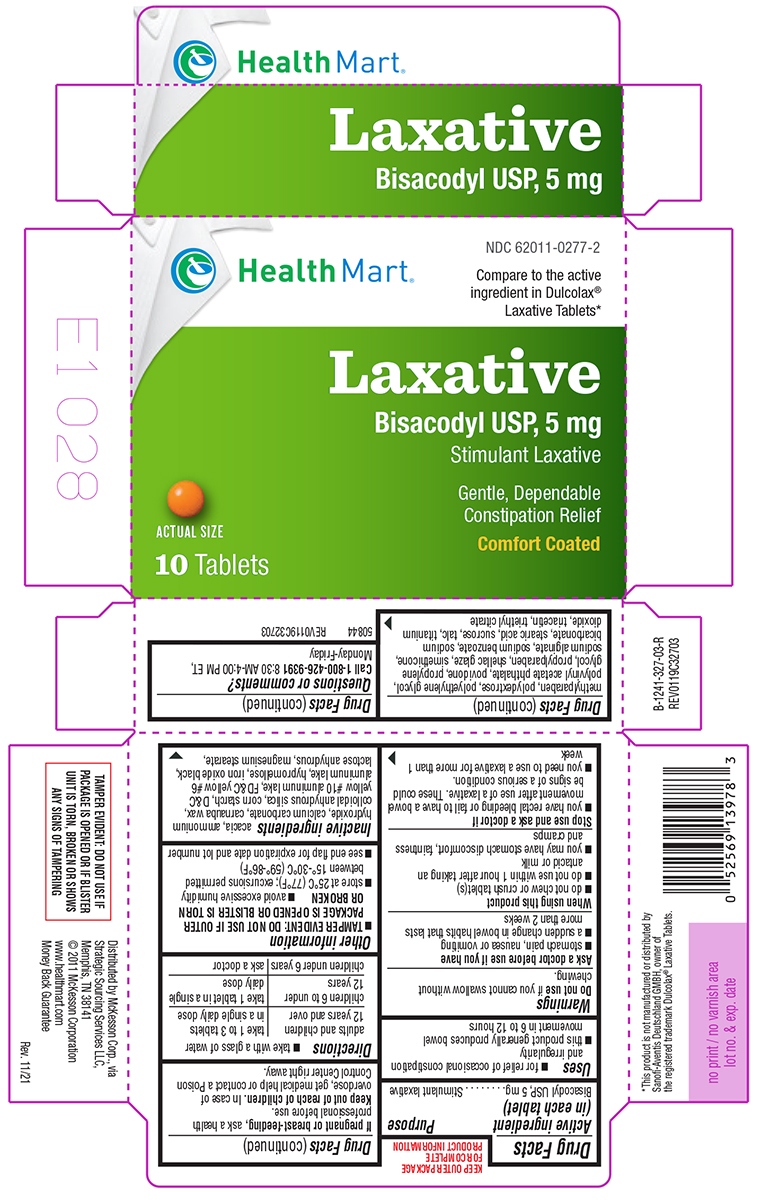
Principal Display Panel
NDC 62011-0277-2
Health Mart®
Compare to the active
ingredient in Dulcolax®
Laxative Tablets*
Laxative
Bisacodyl USP, 5 mg
Stimulant Laxative
Gentle, Dependable
Constipation Relief
Comfort Coated
Actual Size
10 Tablets
TAMPER EVIDENT: DO NOT USE IF
PACKAGE IS OPENED OR IF BLISTER
UNIT IS TORN, BROKEN OR SHOWS
ANY SIGNS OF TAMPERING
This product is not manufactured or
distributed by Sanofi-Aventis Deutschland
GMBH, owner of the registered trademark
Dulcolax® Laxative Tablets.
50844 REV0119C32703
Distributed by McKesson Corp., via
Strategic Sourcing Services LLC,
Memphis, TN 38141
©2011 McKesson Corporation
www.healthmart.com
Money Back Guarantee
Rev.11/21
Health mart 44-327