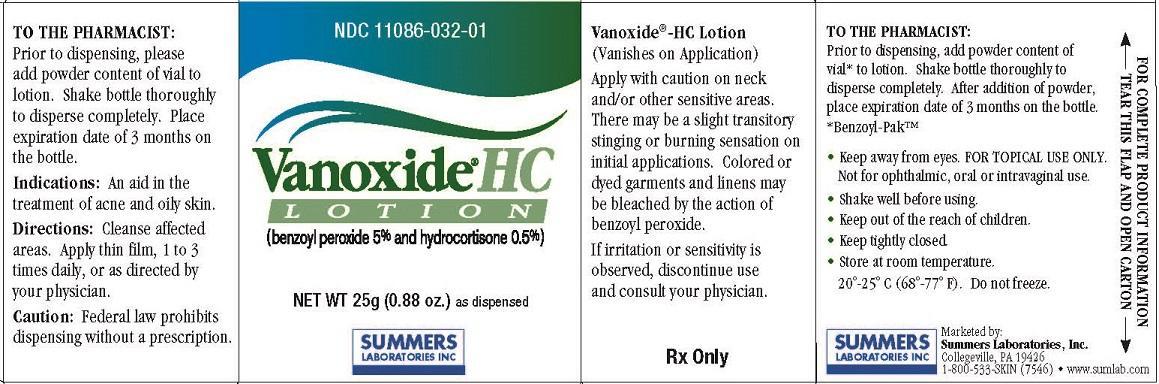
Apply with caution on neck and/or other sensitive areas. There may be a slight transitory stinging or burning sensation on initial applications. Colored or dyed garments and linens may be bleached by the action of benzoyl peroxide. If irritation or sensitivity is
observed, discontinue use and consult your physician.
TO THE PHARMACIST:
Prior to dispensing, add powder content of vial* to lotion. Shake bottle thoroughly to disperse completely. After addition of powder, place expiration date of 3 months on the bottle.
*Benzoyl-Pak™