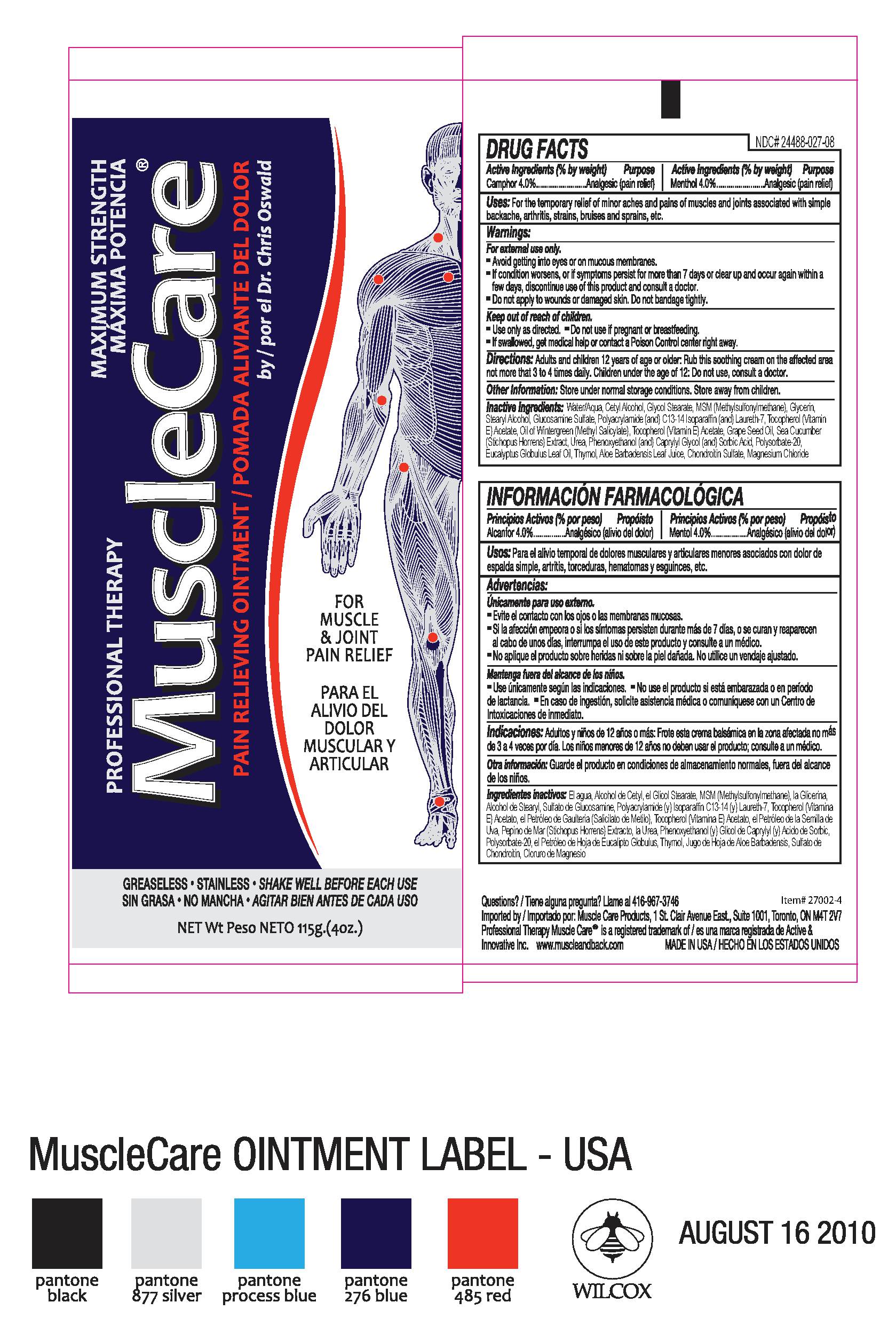
Active Ingredietns (% by weight) Purpose
Camphor 4%.........................Analgesic (pain relief)
Menthol 4%...........................Analgesic (pain relief)
Uses:
For the temporary relief of minor aches and pains of muscles and joints associated with simple backache, arthritis, strains, bruises and sprains, etc.
Warnings:
For external use only.
- Avoid getting into eyes or on mucous membranes.
- If condition worsens, or if symptoms persist for more than 7 days or clear up and occur again within a few days, discontinue use of this product and consult a doctor.
- Do not apply to wounds or damaged skin. Do not bandage tightly.
Keep out of reach of children.
- Use only as directed
- Do not use if pregnant or breastfeeding
- If swallowed, get medical help or contact a Poison Control center right away.
Directions: Adults and children 12 years of age or older: Rub this soothing cream on the affected area not more than 3 to 4 times daily. Children under the age of 12: Do not use, consult a doctor.
Inactive Ingredients: Water/Aqua, Cetyl Alcohol, Glycol Stearate, MSM (methylsulfonylmethane), Glycerin,
Stearyl Alcohol, Glucosamine Sulfate, Polyacrylamide (and) C13-14 Isoparaffin (and) Laureth-7, Tocopherol (Vitamin E) Acetate, Oil of Wintergreen (Methyl Salicylate), Grape Seed Oil, Sea Cucumber (Stichopus Horrens) Extract, Urea, Phenoxyethanol (and) Caprylyl Glycol (and) Sorbic Acid, Polysorbate-20, Eucalyptus Globulus Leaf Oil, Thymol, Aloe Barbadensis Leaf Juice, Chondroitin Sulfate, Magnesium Chloride