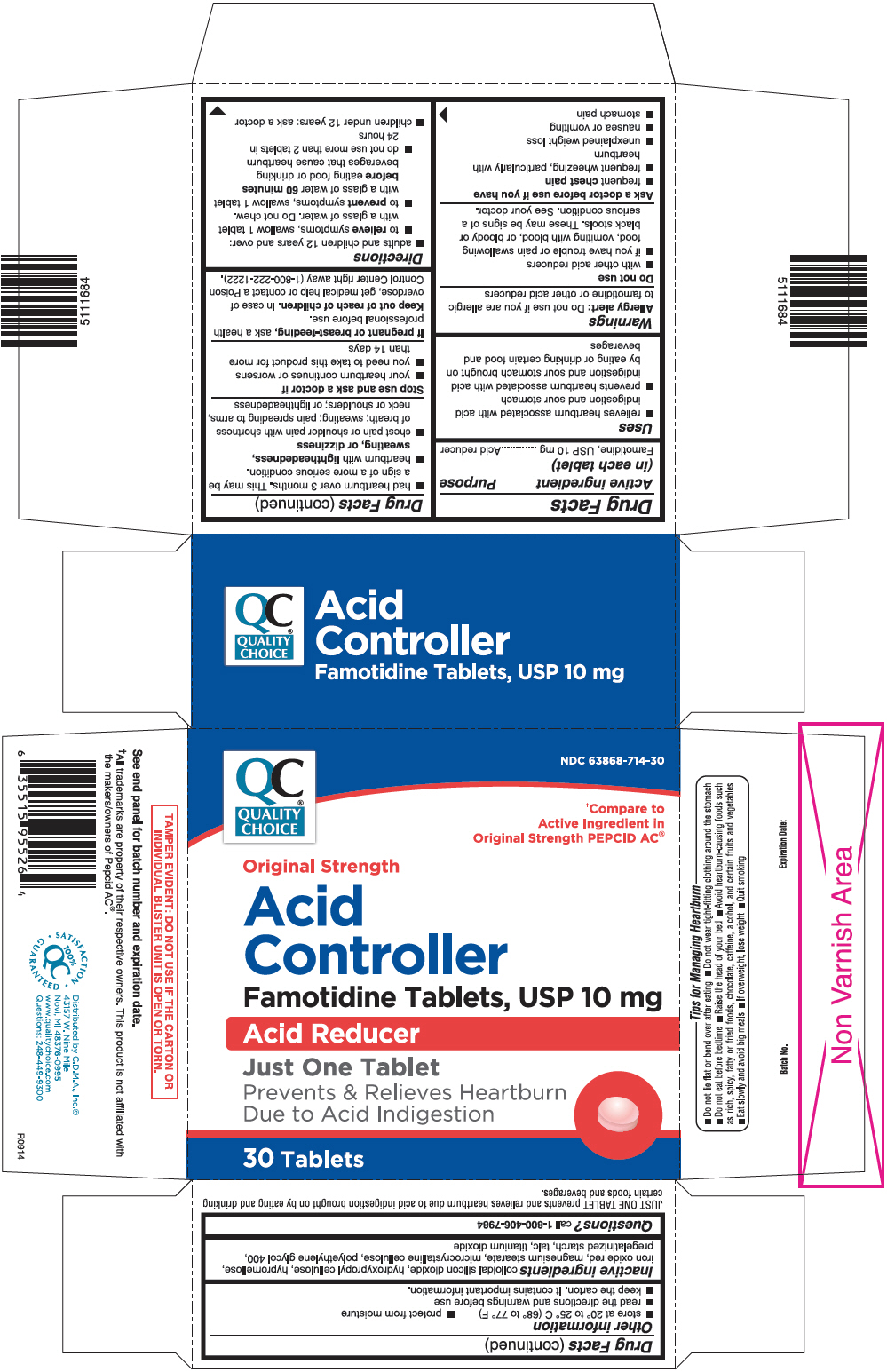
USES
- relieves heartburn associated with acid indigestion and sour stomach
- prevents heartburn associated with acid indigestion and sour stomach brought on by eating or drinking certain food and beverages
WARNINGS
Allergy alert: Do not use if you are allergic to famotidine or other acid reducers
Do not use
- with other acid reducers
- if you have trouble or pain swallowing food, vomiting with blood, or bloody or black stools. These may be signs of a serious condition. See your doctor.
- with other acid reducers
- if you have kidney disease, except under the advice and supervision of a doctor.
- if you have trouble or pain swallowing food, vomiting with blood, or bloody or black stools. These may be signs of a serious condition. See your doctor.
Ask a doctor before use if you have
- frequent chest pain
- frequent wheezing, particularly with heartburn
- unexplained weight loss
- nausea or vomiting
- stomach pain
- had heartburn over 3 months. This may be a sign of a more serious condition.
- heartburn with lightheadedness, sweating, or dizziness
- chest pain or shoulder pain with shortness of breath; sweating; pain spreading to arms, neck or shoulders; or lightheadedness
DIRECTIONS
- adults and children 12 years and over:
- to relieve symptoms, swallow 1 tablet with a glass of water. Do not chew.
- to prevent symptoms, swallow 1 tablet with a glass of water 60 minutes before eating food or drinking beverages that cause heartburn
- do not use more than 2 tablets in 24 hours
- children under 12 years: ask a doctor
- adults and children 12 years and over:
- to relieve symptoms, swallow 1 tablet with a glass of water. Do not chew.
- to prevent symptoms, swallow 1 tablet with a glass of water at any time from 10 to60 minutes before eating food or drinking beverages that cause heartburn
- do not use more than 2 tablets in 24 hours
- children under 12 years: ask a doctor
OTHER INFORMATION
- store at 20° to 25°C (68° to 77°F)
- protect from moisture
- read the directions and warnings before use
- keep the carton. It contains important information.
- TAMPER EVIDENT: DO NOT USE IF THE CARTON OR INDIVIDUAL BLISTER UNIT IS OPEN OR TORN.
- TAMPER EVIDENT: DO NOT USE IF THE CARTON OR PRINTED FOIL UNDER CAP IS OPEN OR TORN.
INACTIVE INGREDIENTS
Colloidal silicon dioxide, hydroxypropyl cellulose, hypromellose, iron oxide red (only for Famotidine USP, 10 mg), magnesium stearate, microcrystalline cellulose, polyethylene glycol 400, pregelatinized starch, talc, titanium dioxide
PATIENT INFORMATION
- 1 tablet relieves heartburn due to acid indigestion
- Famotidine prevents heartburn due to acid indigestion brought on by eating and drinking certain foods and beverages.
- Do not lie flat or bend over after eating
- Do not wear tight-fitting clothing around the stomach
- Do not eat before bedtime
- Raise the head of your bed
- Avoid heartburn - causing foods such as rich, spicy, fatty or fried foods, chocolate, caffeine, alcohol, and certain fruits and vegetables
- Eat slowly and avoid big meals
- If overweight, lose weight
- Quit smoking
PRINCIPAL DISPLAY PANEL - 10 mg Tablet Blister Pack Carton
QUALITY®
CHOICE
NDC 63868-714-30
†Compare to
Active Ingredient in
Original Strength PEPCID AC®
Original Strength
Acid
Controller
Famotidine Tablets, USP 10 mg
Acid Reducer
Just One Tablet
Prevents & Relieves Heartburn
Due to Acid Indigestion
30 Tablets
PRINCIPAL DISPLAY PANEL - 20 mg Tablet Bottle Carton
NDC 63868-486-25
QUALITY®
CHOICE
†Compare to
Active Ingredient in
Maximum Strength PEPCID AC®
Maximum Strength
Acid
Controller
Famotidine Tablets, USP 20 mg
Acid Reducer
Just One Tablet
Prevents & Relieves Heartburn
Due to Acid Indigestion
25 Tablets
