Uses
- •
- relieves occasional constipation (irregularity)
- •
- generally produces bowel movement in 12 to 72 hours
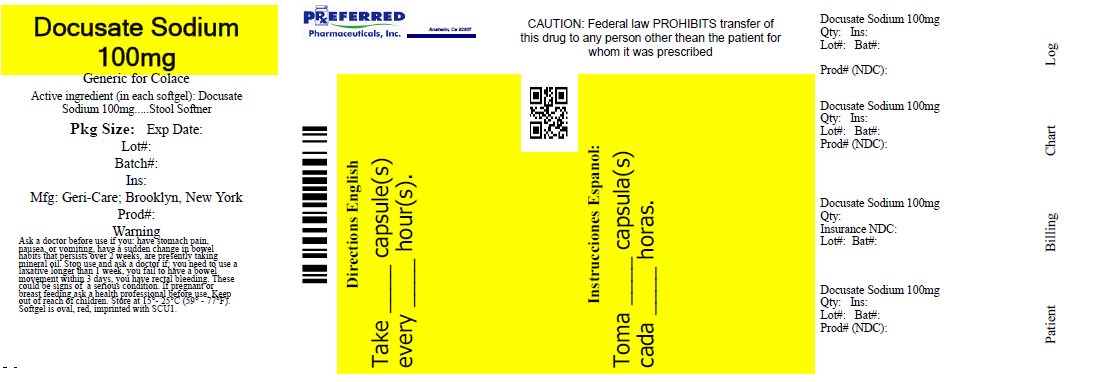
Warnings
Ask a doctor before use if you
• have stomach pain, nausea or vomiting
• have a sudden change in bowel habits that persists over a period of 2 weeks
• are presently taking mineral oil
Stop use and ask a doctor if
• you need to use a laxative longer than 1 week
• you have rectal bleeding or fail to have a bowel movement. These
could be signs of a serious condition.
If pregnant or breast-feeding, ask a health professional before use.
Directions
• do not exceed recommended dose
• adults and children 12 years and older: take 1-3
softgels daily until first bowel movement; 1 softgel
daily thereafter, or as directed by a doctor
• children under 12: consult a doctor
Other information
• each softgel contains: sodium 7 mg. Very low sodium
• store at 59°-77°F (15°-25°C)
• keep tightly closed
• Tamper Evident: Do not use if imprinted seal
under cap is missing or broken.
Repackaged By: Preferred Pharmaceuticals Inc.