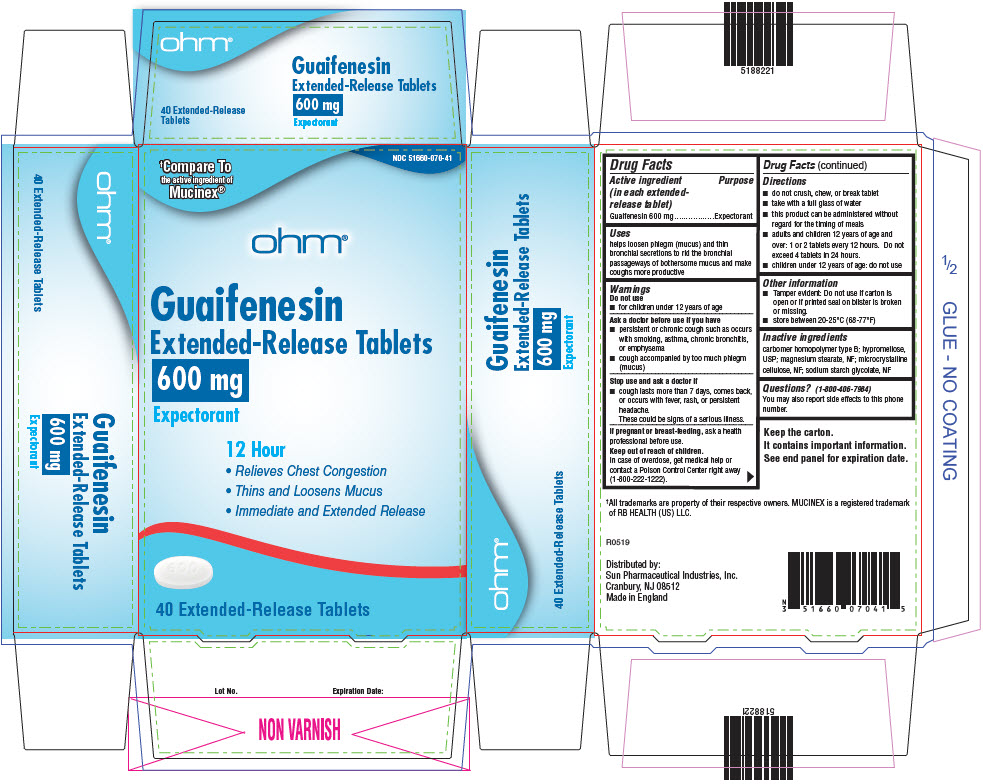
Active ingredient (in each extended-release tablet)
Guaifenesin 600 mg
Uses
helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus and make coughs more productive
Warnings
Do not use
- •
- for children under 12 years of age
Ask a doctor before use if you have
- •
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
- •
- cough accompanied by too much phlegm (mucus)
Stop use and ask a doctor if
- •
- cough lasts more than 7 days, comes back, or occurs with fever, rash, or persistent headache.
These could be signs of a serious illness.
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away (1-800-222-1222).
Directions
- •
- do not crush, chew, or break tablet
- •
- take with a full glass of water
- •
- this product can be administered without regard for the timing of meals
- •
- adults and children 12 years of age and over: 1 or 2 tablets every 12 hours. Do not exceed 4 tablets in 24 hours.
- •
- children under 12 years of age: do not use
Other information
- •
- Tamper evident: Do not use if carton is open or if printed seal on blister is broken or missing.
- •
- store between 20-25°C (68-77°F)
Inactive ingredients
carbomer homopolymer type B; hypromellose, USP; magnesium stearate, NF; microcrystalline cellulose, NF; sodium starch glycolate, NF
Questions?
(1-800-406-7984)
You may also report side effects to this phone number.
Distributed by:
Sun Pharmaceutical Industries, Inc.
Cranbury, NJ 08512
PRINCIPAL DISPLAY PANEL - 600 mg Tablet Blister Pack Carton
NDC 51660-070-41
†Compare To
the active ingredient of
Mucinex®
ohm®
Guaifenesin
Extended-Release Tablets
600 mg
Expectorant
12 Hour
- Relieves Chest Congestion
- Thins and Loosens Mucus
- Immediate and Extended Release
40 Extended-Release Tablets