DICLOFENAC SODIUM DELAYED-RELEASE- diclofenac sodium tablet, delayed release
ALPHAPHARM PTY LTD
Reference Label Set Id: 76bc3d4e-95ec-44cc-b7fd-aaf8b4bd7f14
----------
Diclofenac Sodium Delayed-releaseTablets USP
DESCRIPTION
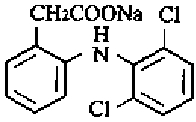
Diclofenac, as the sodium salt, is a benzeneacetic acid derivative, designated chemically as Sodium [o-(2,6- dichloroanillino)phenyl]acetate. The structural formula is shown in Figure 1.
Molecular Formula: C14H10Cl2NNaO2
Molecular Weight: 318.13
Diclofenac sodium, is a faintly yellowish white to light beige, virtually odorless, slightly hygroscopic crystalline powder. It is freely soluble in methanol, soluble in ethanol, and practically insoluble in chloroform and in dilute acid. Diclofenac sodium is sparingly soluble in water. The n-octanol/water partition coefficient is 13.4 at pH 7.4 and 1545 at pH 5.2. The sodium salt has a single dissociation constant (pKa) of 4.0±0.2 at 25°C in water.
Each delayed-release tablet for oral administration contains 50 mg or 75 mg of diclofenac sodium. In addition, each tablet contains the following inactive ingredients: crospovidone, FD & C Blue No. 2 Aluminium Lake (50 mg), FD & C Red No. 40 (75 mg), FD & C Yellow No. 6, hydroxy propyl methyl cellulose, iron oxide yellow (50 mg), lactose monohydrate, magnesium stearate, methacrylic acid copolymer, microcrystalline cellulose, polyethylene glycol, povidone, simethicone, sodium hydroxide, talc and titanium dioxide.
CLINICAL PHARMACOLOGY
Pharmacodynamics
Diclofenac is a nonsteroidal anti-inflammatory drug (NSAID). In pharmacologic studies, diclofenac has shown anti-inflammatory, analgesic, and antipyretic activity. As with other NSAIDs, its mode of action is not known; its ability to inhibit prostaglandin synthesis, however, may be involved in its anti-inflammatory activity.
Pharmacokinetics
Diclofenac sodium delayed-release tablets are in a pharmaceutical formulation that resists dissolution in the low pH of gastric fluid but allows a rapid release of drug in the higher pH-environment in the duodenum. Its pattern of drug release and absorption is shown in Table 1.
|
Drug |
Dose |
AUC |
Cmax (ng/mL) |
Tmax (hr) |
|
Diclofenac |
50 |
1429 |
1417 |
2.22 |
Absorption
Under fasting condition, diclofenac is completely absorbed from the gastrointestinal tract. However, due to first-pass metabolism, only about 50% of the absorbed dose is systemically available.
Peak plasma levels are achieved in 2 hours in fasting normal volunteers, with a range from 1 to 4 hours. The area-under-the-plasma-concentration curve (AUC) is dose-proportional within the range of 25 mg to 150 mg. Peak plasma levels are less than dose-proportional and are approximately 1.0, 1.5, and 2.0 µg/mL for 25 mg, 50 mg, and 75 mg doses, respectively. It should be noted that the administration of several individual diclofenac sodium tablets may not yield equivalent results in peak concentration as the administration of one tablet of a higher strength. This is probably due to the staggered gastric emptying of tablets into the duodenum. After repeated oral administration of diclofenac sodium 50 mg b.i.d., diclofenac did not accumulate in plasma.
When diclofenac sodium is taken with food, there is usually a delay in the onset of absorption of 1 to 4.5 hours, with delays as long as 10 hours in some patients, and a reduction in peak plasma levels of approximately 40%. The extent of absorption of diclofenac, however, is not significantly affected by food intake.
Distribution
Plasma concentrations of diclofenac decline from peak levels in a biexponential fashion, with the terminal phase having a half-life of approximately 2 hours. Clearance and volume of distribution are about 350 mL/min and 650 mL/kg, respectively. More than 99% of diclofenac is reversibly bound to human plasma albumin.
As with other NSAIDs, diclofenac diffuses into and out of the synovial fluid. Diffusion into the joint occurs when plasma levels are higher than those in the synovial fluid, after which the process reverses and synovial fluid levels are higher than plasma levels. It is not known whether diffusion into the joint plays a role in the effectiveness of diclofenac.
Metabolism and Elimination
Diclofenac is eliminated through metabolism and subsequent urinary and biliary excretion of the glucuronide and the sulfate conjugates of the metabolites. Approximately 65% of the dose is excreted in the urine, and approximately 35% in the bile.
Conjugates of unchanged diclofenac account for 5% to 10% of the dose excreted in the urine and for less than 5% excreted in the bile. Little or no unchanged unconjugated drug is excreted. Conjugates of the principal metabolite account for 20% to 30% of the dose excreted in the urine and for 10% to 20% of the dose excreted in the bile. Conjugates of three other metabolites together account for 10% to 20% of the dose excreted in the urine and for small amounts excreted in the bile. The elimination half-life values for these metabolites are shorter than those for the parent drug. Urinary excretion of an additional metabolite (half-life 80 hours) accounts for only 1.4% of the oral dose. The degree of accumulation of diclofenac metabolites is unknown. Some of the metabolites may have activity.
Special Populations
A 4-week study, comparing plasma level profiles of diclofenac (diclofenac sodium 50 mg b.i.d.) in younger (26 to 46 years) versus older (66 to 81 years) adults, did not show differences between age groups (10 patients per age group).
Patients with Renal and/or Hepatic Impairment:
To date, no differences in the pharmacokinetics of diclofenac have been detected in studies of patients with renal (50 mg intravenously) or hepatic impairment (100 mg oral solution). In patients with renal impairment (N=5, creatinine clearance 3 to 42 mL/min), AUC values and elimination rates were comparable to those in healthy subjects. In patients with biopsy-confirmed cirrhosis or chronic active hepatitis (variably elevated transaminases and mildly elevated billrubins, N=10), diclofenac concentrations and urinary elimination values were comparable to those in healthy subjects.
Clinical Studies
Osteoarthritis:
Diclofenac sodium was evaluated for the management of the signs and symptoms of osteoarthritis of the hip or knee in a total of 633 patients treated for up to 3 months in placebo- and active-controlled clinical trials against aspirin (N=449), and naproxen (N=92). Diclofenac sodium was given both in variable (100 to 150 mg/day) and fixed (150 mg/day) dosing schedules in either b.i.d. or t.i.d. dosing regimens. In these trials, diclofenac sodium was found to be comparable to 2400 to 3600 mg/day of aspirin or 500 mg/day of naproxen. Diclofenac sodium was effective when administered as either b.i.d. or t.i.d. dosing regimens.
Rheumatoid Arthritis:
Diclofenac sodium was evaluated for managing the signs and symptoms of rheumatoid arthritis in a total of 468 patients treated for up to 3 months in placebo- and active-controlled clinical trials against aspirin (N=290), and ibuprofen (N=74). Diclofenac sodium was given in a fixed (150 or 200 mg/day) dosing schedule as either b.i.d. or t.i.d. dosing regimens. Diclofenac sodium was found to be comparable to 3600 to 4800 mg/day of aspirin, and 2400 mg/day of ibuprofen. Diclofenac sodium was used b.i.d. or t.i.d., administering 150 mg/day in most trials, but 50 mg q.i.d. (200 mg/day) was also studied.
Ankylosing Spondylitis:
Diclofenac sodium was evaluated for the management of the signs and symptoms of ankylosing spondylitis in a total of 132 patients in one active-controlled clinical trial against indomethacin (N=130). Both diclofenac sodium and indomethacin patients were started on 25 mg t.i.d. and were permitted to increase the dose 25 mg/day each week to a maximum dose of 125 mg/day. Diclofenac sodium 75 to 125 mg/day was found to be comparable to indomethacin 75 to 125 mg/day.
Special Studies
(The clinical significance of the findings outlined below is unknown).
G.I.Blood Loss/Endoscopy Data:
G.I. blood loss and endoscopy studies were performed with diclofenac sodium delayed-release tablets that, unlike Immediate-Release Tablets, do not dissolve in the stomach where the endoscopic lesions are primarily seen. A repeat-dose endoscopy study, in patients with rheumatoid arthritis or osteoarthritis treated with diclofenac sodium delayed-release tablets 75 mg – b.i.d. (N=101), or naproxen (immediate-release tablets) 500 mg b.i.d. (N=103) for 3 months, resulted in a significantly smaller number of patients with an increase in endoscopy score from baseline and a significantly lower mean endoscopy score after treatment in the diclofenac sodium-treated patients. Two repeat-dose endoscopic studies, in normal volunteers showed that daily doses of diclofenac sodium delayed-release tablets 75 or 100 mg (N=6 and N=14, respectively) for 1 week caused fewer gastric lesions, and those that did occur had lower scores than those observed following daily 500 mg doses of naproxen (immediate-release tablets). In healthy subjects, the daily administration of 150 mg of diclofenac sodium (N=8) for 3 weeks resulted in a mean fecal blood loss less than that observed with 3.0 g of aspirin daily (N=8). In four repeat-dose studies, mean fecal blood loss with 150 mg of diclofenac sodium was also less than that observed with 750 mg of naproxen (N=8 and N=6) or 150 mg of indomethacin (N=8 and N=6).
INDIVIDUALIZATION OF DOSAGE
Diclofenac, like other NSAIDs, shows interindividual differences in both pharmacokinetics and clinical response (pharmacodynamics). Consequently, the recommended strategy for initiating therapy is to use a starting dose likely to be effective for the majority of patients and to adjust dosage thereafter based on observation of diclofenac’s beneficial and adverse effects.
In patients weighing less than 60 kg (132 lb), or where the severity of the disease, concomitant medication, or other diseases warrant, the maximum recommended total daily dose of diclofenac sodium should be reduced. Experience with other NSAIDs has shown that starting therapy with maximum doses in patients at increased risk due to renal or hepatic disease, low body weight (<60 kg), advanced age, a known ulcer diathesis, or known sensitivity to NSAID effects, is likely to increase frequency of adverse reactions and is not recommended (see PRECAUTIONS).
Osteoarthritis/Rheumatoid Arthritis/Ankylosing Spondylitis:
The usual starting dose of diclofenac sodium delayed-release tablets for patients with osteoarthritis, is 100 to 150 mg/day, using a b.i.d. or t.i.d. dosing regimen. In two variable-dose clinical trials in osteoarthritis using diclofenac sodium delayed-release tablets, of 266 patients started on 100 mg/day, 176 chose to increase the dose to 150 mg/day. Dosages above 200 mg/day have not been studied in patients with osteoarthritis.
The usual starting dose of diclofenac sodium delayed-release tablets for most patients with rheumatoid arthritis is 150 mg/day, using a b.i.d. or t.i.d. dosing regimen. Patients requiring more relief of pain and inflammation may increase the dose to 200 mg/day. In clinical trials, patients receiving 200 mg/day were less likely to drop from the trial due to lack of efficacy than patients receiving 150 mg/day. Dosages above 225 mg/day are not recommended in patients with rheumatoid arthritis because of increased risk of adverse events.
The recommended dose of diclofenac sodium delayed-release tablets for patients with ankylosing spondylitis is 100 to 125 mg/day, using a q.i.d.dosing regimen (see DOSAGE AND ADMINISTRATION regarding the 125 mg/day dosing regimen). In a variable-dose clinical trial, of 132 patients started on 75 mg/day, 122 chose to increase the dose to 125 mg/day. Dosages above 125 mg/day have not been studied in patients with ankylosing spondylitis.
INDICATIONS AND USAGE
Diclofenac sodium delayed-release tablets are indicated for the acute and chronic treatment of signs and symptoms of rheumatoid arthritis, osteoarthritis, and ankylosing spondylitis.
CONTRAINDICATIONS
Diclofenac sodium is contraindicated in patients with known hypersensitivity to diclofenac and diclofenac-containing products. Diclofenac should not be given to patients who have experienced asthma, urticaria, or other allergic-type reactions after taking aspirin or other NSAIDs. Severe, rarely fatal, anaphylactic-like reactions to diclofenac have been reported in such patients (see WARNINGS-Anaphylactoid Reactions, and PRECAUTIONS-Preexisting Asthma).
WARNINGS
Gastrointestinal Effects
Peptic ulceration and gastrointestinal bleeding have been reported in patients receiving diclofenac. Physicians and patients should therefore remain alert for ulceration and bleeding in patients treated chronically with diclofenac even in the absence of previous G.I. tract symptoms. It is recommended that patients be maintained on the lowest dose of diclofenac possible, consistent with achieving a satisfactory therapeutic response.
Risk of G.I. Ulcerations, Bleeding, and Perforation with NSAID Therapy:
Serious gastrointestinal toxicity such as bleeding, ulceration, and perforation can occur at any time, with or without warning symptoms, in patients treated chronically with NSAID therapy. Although minor upper gastrointestinal problems, such as dyspepsia, are common, usually developing early in therapy, physicians should remain alert for ulceration and bleeding in patients treated chronically with NSAIDs even in the absence of previous G.I. tract symptoms. In patients observed in clinical trials of several months to 2 years’ duration, symptomatic upper G.I. ulcers, gross bleeding, or perforation appear to occur in approximately 1% of patients for 3 to 6 months, and in about 2% to 4% of patients treated for 1 year. Physicians should inform patients about the signs and/or symptoms of serious G.I. toxicity and what steps to take if they occur.
Studies to date have not identified any subset of patients not at risk of developing peptic ulceration and bleeding. Except for a prior history of serious G.I. events and other risk factors known to be associated with peptic ulcer disease, such as alcoholism, smoking, etc., no risk factors (e.g., age, sex) have been associated with increased risk. Elderly or debilitated patients seem to tolerate ulceration or bleeding less well than other individuals, and most spontaneous reports of fatal G.I. events are in this population. Studies to date are inconclusive concerning the relative risk of various NSAIDs in causing such reactions. High doses of any NSAID probably carry a greater risk of these reactions, although controlled clinical trials showing this do not exist in most cases. In considering the use of relatively large doses (within the recommended dosage range), sufficient benefit should be anticipated to offset the potential increased risk of G.I. toxicity.
Hepatic Effects
Elevations of one or more liver tests may occur during diclofenac therapy. These laboratory abnormalities may progress, may remain unchanged, or may be transient with continued therapy. Borderline elevations (i.e., less than 3 times the ULN [*the Upper Limit of the Normal range]), or greater elevations of transaminases occurred in about 15% of diclofenac-treated patients. Of the hepatic enzymes, ALT (SGPT) is the one recommended for the monitoring of liver injury.
In clinical trials, meaningful elevations (i.e., more than 3 times the ULN) of AST (SGOT) (ALT was not measured in all studies) occurred in about 2% of approximately 5700 patients at some time during diclofenac sodium treatment. In a large, open, controlled trial, meaningful elevations of ALT and/or AST occurred in about 4% of 3700 patients treated for 2 to 6 months, including marked elevations (i.e., more than 8 times the ULN) in about 1% of the 3700 patients. In that open-label study, a higher incidence of borderline (less than 3 times the ULN), moderate (3 to 8 times the ULN), and marked (>8 times the ULN) elevations of ALT or AST was observed in patients receiving diclofenac when compared to other NSAIDs. Transaminase elevations were seen more frequently in patients with osteoarthritis than in those with rheumatoid arthritis (see ADVERSE REACTIONS).
In addition to enzyme elevations seen in clinical trials, postmarketing surveillance has found rare cases of severe hepatic reactions, including liver nacrosis, jaundice, and fulminant fatal hepatitis with and without jaundice. Some of these rare reported cases underwent liver transplantation.
Physicians should measure transaminases periodically in patients receiving long-term therapy with diclofenac, because severe hepatotoxicity may develop without a prodrome of distinguishing symptoms. The optimum times for making the first and subsequent transaminase measurements are not known. In the largest U.S. trial (open-label) that involved 3700 patients monitored first at 8 weeks and 1200 patients monitored again at 24 weeks, almost all meaningful elevations in transaminases were detected before patients became symptomatic. In 42 of the 51 patients in all trials who developed marked transaminase elevations, abnormal tests occurred during the first 2 months of therapy with diclofenac. Postmarketing experience has shown severe hepatic reactions can occur at any time during treatment with diclofenac. Cases of drug-induced hepatotoxicity have been reported in the first month, and in some cases, the first two months of therapy. Based on these experiences, transaminases should be monitored within 4 to 8 weeks after initiating treatment with diclofenac (see PRECAUTIONS-Laboratory Tests). As with other NSAIDs, if abnormal liver tests persist or worsen, if clinical signs and/or symptoms consistent with liver disease develop, or if systemic manifestations occur (e.g., eosinophilla, rash, etc.), diclofenac should be discontinued immediately.
To minimize the possibility that hepatic injury will become severe between transaminase measurements, physicians should inform patients of the warning signs and symptoms of hepatotoxicity (e.g., nausea, fatigue, lethargy, pruritus, jaundice, right upper quadrant tenderness, and “flu-like” symptoms), and the appropriate action patients should take if these signs and symptoms appear.
Anaphylactoid Reactions
As with other NSAIDs, anaphylactoid reactions may occur in patients without prior exposure to diclofenac. Diclofenac should not be given to patients with the aspirin triad. The triad typically occurs in asthmatic patients who experience rhinitis with or without nasal polyps, or who exhibit severe, potentially fatal bronchospasm after taking aspirin or other nonsteroidal anti-inflammatory drugs. Fatal reactions have been reported in such patients (see CONTRAINDICATIONS, and PRECAUTIONS-Preexisting Asthma). Emergency help should be sought in cases where an anaphylactoid reaction occurs.
PRECAUTIONS
General
Diclofenac sodium delayed-release tablets should not be used concomitantly with other diclofenac-containing products since they also circulate in plasma as the diclofenac anion.
Fluid Retention and Edema:
Fluid retention and edema have been observed in some patients taking diclofenac. Therefore, as with other NSAIDs, diclofenac should be used with caution in patients with a history of cardiac decompensation, hypertension, or other conditions predisposing to fluid retention.
Hematologic Effects:
Anemia is sometimes seen in patients receiving diclofenac or other NSAIDs. This may be due to fluid retention, G.I. blood loss, or an incompletely described effect upon erythropoiesis.
Renal Effects:
As a class, NSAIDs have been associated with renal papiliary necrosis and other abnormal renal pathology in long-term administration to animals. In oral diclofenac studies in animals, some evidence of renal toxicity was noted. Isolated incidents of papiliary necrosis were observed in a few animals at high doses (20 to 120 mg/kg) in several baboon subacute studies. In patients treated with diclofenac, rare cases of interstitial nephritis and papiliary necrosis have been reported (see ADVERSE REACTIONS).
A second form of renal toxicity, generally associated with NSAIDs, is seen in patients with conditions leading to a reduction in renal blood flow or blood volume, where renal prostaglandins have a supportive role in the maintenance of renal perfusion. In these patients, administration of an NSAID results in a dose-dependent decrease in prostaglandin synthesis and, secondarily, in a reduction of renal blood flow, which may precipitate overt renal failure. Patients at greatest risk of this reaction are those with impaired renal function, heart failure, liver dysfunction, those taking diuretics, and the elderly. Discontinuation of NSAID therapy is typically followed by recovery to the pretreatment state.
Cases of significant renal failure in patients receiving diclofenac have been reported from marketing experience, but were not observed in over 4000 patients in clinical trials during which serum creatinine and BUN values were followed serially. There were only 11 patients (0.3%) whose serum creatinine and concurrent serum BUN values were greater than 2.0 mg/dL, and 40 mg/dL, respectively, while on diclofenac (mean rise in the 11 patients: creatinine 2.3 mg/dL and BUN 28.4 mg/dL).
Since diclofenac metabolites are eliminated primarily by the kidneys, patients with significantly impaired renal function should be more closely monitored than subjects with normal renal function.
Porphyria:
The use of diclofenac in patients with hepatic porphyria should be avoided. To date, 1 patient has been described in whom diclofenac probably triggered a clinical attack of porphyria. The postulated mechanism, demonstrated in rats, for causing such attacks by diclofenac, as well as some other NSAIDs, is through stimulation of the porphyrin precursor delta-aminolevulinic acid (ALA).
Aseptic Meningitis:
As with other NSAIDs, aseptic meningitis with fever and coma has been observed on rare occasions in patients on diclofenac therapy. Although it is probably more likely to occur in patients with systemic lupus erythematosus and related connective tissue diseases, it has been reported in patients who do not have an underlying chronic disease. If signs or symptoms of meningitis develop in a patient on diclofenac, the possibility of its being related to diclofenac should be considered.
Preexisting Asthma:
About 10% of patients with asthma may have aspirin-sensitive asthma. The use of aspirin in patients with aspirin-sensitive asthma has been associated with severe bronchospasm which can be fatal. Since cross-reactivity, including bronchospasm, between aspirin and other nonsteroidal anti-inflammatory drugs has been reported in such aspirin-sensitive patients, diclofenac should not be administered to patients with this form of aspirin sensitivity and should be used with caution in all patients with preexisting asthma.
Other Precautions:
The pharmacologic activity of diclofenac may reduce fever and inflammation, thus diminishing their utility as diagnostic signs in detecting underlying conditions.
In order to avoid exacerbation of manifestations of adrenal insufficiency, patients who have been on prolonged corticosteroid treatment should have their therapy tapered slowly rather than discontinued abruptly when diclofenac is added to the treatment program.
Blurred and/or diminished vision, scotomata, and/or changes in color vision have been reported. If a patient develops such complaints while receiving diclofenac, the drug should be discontinued and the patient should have an ophthalmologic examination which includes central visual fields and color vision testing.
Information for Patients
Diclofenac, like other drugs of its class, is not free of side effects. The side effects of these drugs can cause discomfort and, rarely, more serious side effects, such as gastrointestinal bleeding, and more rarely, liver toxicity (see WARNINGS, Hepatic Effects), which may result in hospitalization and even fatal outcomes.
NSAIDs are often essential agents in the management of arthritis and have a major role in the management of pain, but they also may be commonly employed for conditions that are less serious.
Physicians may wish to discuss with their patients the potential risks (see WARNINGS, PRECAUTIONS, and ADVERSE REACTIONS) and likely benefits of NSAID treatment, particularly when the drugs are used for less serious conditions where treatment without NSAIDs may represent an acceptable alternative to both the patient and physician.
Because serious G.I. tract ulceration and bleeding can occur without warning symptoms, physicians should follow chronically treated patients for the signs and symptoms of ulcerations and bleeding and should inform them of the importance of this follow-up (see WARNINGS, Gastrointestinal Effects, Risk of G.I. Ulcerations, Bleeding and Perforation with NSAID Therapy). If diclofenac is used chronically, patients should also be instructed to report any signs and symptoms that might be due to hepatotoxicity of diclofenac; these symptoms may become evident between visits when periodic liver laboratory tests are performed (see WARNINGS, Hepatic Effects, and PRECAUTIONS-Laboratory Tests).
Laboratory Tests
Hepatic Effects:
Transaminases and other hepatic enzymes should be monitored in patients treated with NSAIDs. For patients on diclofenac therapy, it is, recommended that a determination be made within 4 weeks of initiating therapy, and at intervals thereafter. If clinical signs and symptoms consistent with liver disease develop, or if systemic manifestations occur (e.g. eosinophilia, rash, etc.) and abnormal liver tests are detected, persist or worsen, diclofenac should be discontinued immediately.
Drug Interactions
Aspirin:
Concomitant administration of diclofenac and aspirin is not recommended because diclofenac is displaced from its binding sites during the concomitant administration of aspirin, resulting in lower plasma concentrations, peak plasma levels, and AUC values.
Anticoagulants:
While studies have not shown diclofenac to interact with anticoagulants of the warfarin type, caution should be exercised, nonetheless, since interactions have been seen with other NSAIDs. Because prostaglandins play an important role in hemostasis, and NSAIDs affect platelet function as well, concurrent therapy with all NSAIDs, including diclofenac, and warfarin requires close monitoring of patients to be certain that no change in their anticoagulant dosage is required.
Digoxin, Methotrexate, Cyclosporine:
Diclofenac, like other NSAIDs, may affect renal prostaglandins and increase the toxicity of certain drugs. Ingestion of diclofenac may increase serum concentrations of digoxin and methotrexate and increase cyclosporine's nephrotoxicity. Patients who begin taking diclofenac or who increase their diclofenac dose or any other NSAID while taking digoxin, methotrexate, or cyclosporine may develop toxicity characteristics for these drugs. They should be observed closely, particularly if renal function is impaired. In the case of digoxin, serum levels should be monitored.
Lithium:
Diclofenac decreases lithium renal clearance and increases lithium plasma levels. In patients taking diclofenac and lithium concomitantly, lithium toxicity may develop.
Oral Hypoglycemics:
Diclofenac does not alter glucose metabolism in normal subjects nor does it alter the effects of oral hypoglycemic agents. There are rare reports, however, from marketing experiences, of changes in effects of insulin or oral hypoglycemic agents in the presence of diclofenac that necessitated changes in the doses of such agents. Both hypo-and hyperglycemic effects have been reported. A direct causal relationship has not been established, but physicians should consider the possibility that diclofenac may alter a diabetic patient’s response to insulin or oral hypoglycemic agents.
Diuretics:
Diclofenac and other NSAIDs can inhibit the activity of diuretics. Concomitant treatment with potassium-sparing diuretics may be associated with increased serum potassium levels.
Other Drugs:
In small groups of patients (7 to 10/interaction study), the concomitant administration of azathioprine, gold, chloroquine, D-penicillamine, prednisolone, doxycycline, or digitoxin did not significantly affect the peak levels and AUC values of diclofenac. Phenobarbital toxicity has been reported to have occurred in a patient on chronic phenobarbital treatment following the initiation of diclofenac therapy.
Protein Binding
In vitro, diclofenac interferes minimally or not at all with the protein binding of salicylic acid (20% decrease in binding), tolbutamide, prednisolone (10% decrease in binding), or warfarin. Benzylpenicillin, ampicillin, oxacillin, chlortetracycline, doxycycline, cephalothin, erythromycin, and sulfamethoxazole have no influence in vitro on the protein binding of diclofenac in human serum.
Drug/Laboratory Test Interactions
Effect on Blood Coagulation:
Diclofenac increases platelet aggregation time but does not affect bleeding time, plasma thrombin clotting time, plasma fibrinogen, or factors V and VII to XII. Statistically significant changes in prothrombin and partial thromboplastin times have been reported in normal volunteers. The mean changes were observed to be less than 1 second in both instances, however, and are unlikely to be clinically important. Diclofenac is a prostaglandin synthetase inhibitor, however, and all drugs that inhibit prostaglandin synthesis interfere with platelet function to some degree; therefore, patients who may be adversely affected by such an action should be carefully observed.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term carcinogenicity studies in rats given diclofenac sodium up to 2 mg/kg/day or (12 mg/m2/day, approximately the human dose) have revealed no significant increases in tumor incidence. There was a slight increase in benign mammary fibroadenomas in mid-dose-treated (0.5 mg/kg/day or 3 mg/m2/day) female rats (high-dose females had excessive mortality), but the increase was not significant for this common rat tumor. A 2-year carcinogenicity study conducted in mice employing diclofenac sodium at doses up to 0.3 mg/kg/day (0.9 mg/m2/day) in males and 1 mg/kg/day (3 mg/m2/day) in females did not reveal any oncogenic potential. Diclofenac sodium did not show mutagenic activity in in vitro point mutation assays in mammalian (mouse lymphoma) and microbial (yeast, Ames) test systems and was nonmutagenic in several mammalian in vitro and in vivo tests, including dominant lethal and male germinal epithelial chromosomal studies in mice, and nucleus anomaly and chromosomal aberration studies in Chinese hamsters. Diclofenac sodium administered to male and female rats at 4 mg/kg/day (24 mg/m2/day) did not affect fertility.
Pregnancy, Teratogenic Effects, Pregnancy Category B
Reproduction studies have been performed in mice given diclofenac sodium ( up to 20 mg/kg/day or 60 mg/m2/day) and in rats and rabbits given diclofenac sodium (up to 10 mg/kg/day or 60 mg/m2/day for rats, and 80 mg/m2/day for rabbits), and have revealed no evidence of teratogenicity despite the induction of maternal toxicity and fetal toxicity. In rats, maternally toxic doses were associated with dystocia, prolonged gestation, reduced fetal weights and growth, and reduced fetal survival. Diclofenac has been shown to cross the placental barrier in mice and rats. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should not be used during pregnancy unless the benefits to the mother justify the potential risk to the fetus. Because of the risk to the fetus resulting in premature closure of the ductus arteriosus, diclofenac should be avoided in late pregnancy.
Labor and Delivery
The effects of diclofenac on labor and delivery in pregnant women are unknown. Because of the known effects of prostaglandin-inhibiting drugs on the fetal cardiovascular system (closure of ductus arteriosus), use of diclofenac during late pregnancy should be avoided and, as with other nonsteroidal anti-inflammatory drugs, it is possible that diclofenac may inhibit uterine contractions and delay parturition.
Nursing Mothers
Because of the potential for serious adverse reactions in nursing infants from diclofenac, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
Safety and effectiveness of diclofenac in pediatric patients have not been established.
Geriatric Use
Of the more than 6000 patients treated with diclofenac in U.S. trials, 31% were older than 65 years of age. No overall difference was observed between efficacy, adverse event, or pharmacokinetic profiles of older and younger patients. As with any NSAID, the elderly are likely to tolerate adverse reactions less well than younger patients.
ADVERSE REACTIONS
Adverse reaction information is derived from blinded, controlled and open-label clinical trials, as well as worldwide marketing experience. In the description below, rates of more common events represent clinical study results; rarer events are derived principally form marketing experience and publications, and accurate rate estimates are generally not possible.
The incidence of common adverse reactions (greater than 1%) is based upon controlled clinical trials in 1543 patients treated up to 13 weeks with diclofenac sodium delayed-release tablets. By far the most common adverse effects were gastrointestinal symptoms, most of them minor, occurring in about 20%, and leading to discontinuation in about 3% of patients. Peptic ulcer or G.I. bleeding occurred in clinical trials in 0.6% (95% confidence interval: 0.2% to 1%) of approximately 1800 patients during their first 3 months of diclofenac treatment and in 1.6% (95% confidence interval: 0.8% to 2.4%) of approximately 800 patients followed for 1 year.
Gastrointestinal symptoms were followed in frequency by central nervous system side effects such as headache (7%) and dizziness (3%).
Meaningful (exceeding 3 times the Upper Limit of Normal) elevations of ALT (SGPT) or AST (SGOT) occurred at an overall rate of approximately 2% during the first 2 months of Diclofenac Sodium treatment. Unlike aspirin-related elevations, which occur more frequently in patients with rheumatoid arthritis, these elevations were more frequently observed in patients with osteoarthritis (2.6%) than in patients with rheumatoid arthritis (0.7%). Marked elevations (exceeding 8 times the ULN) were seen in 1% of patients treated for 2 to 6 months (see WARNINGS, Hepatic Effects).
The following adverse reactions were reported in patients treated with diclofenac:
Incidence Greater Than 1% - Causal Relationship Probable:
(All derived from clinical trials.)
*Incidence, 3% to 9% (Incidence of unmarked reactions is 1% to 3%).
Body as a Whole: Abdominal pain or cramps, * headache, * fluid retention, abdominal distention.
Digestive: Diarrhea, * indigestion, * nausea, * constipation, * flatulence, liver test abnormalities, * PUB, i.e., peptic ulcer, with or without bleeding and/or perforation, or bleeding without ulcer (see above and also WARNINGS).
Nervous System: Dizziness.
Skin and Appendages: Rash, pruritus.
Special Senses: Tinnitus.
Incidence Less Than 1% - Causal Relationship Probable:
(Adverse reactions reported only in worldwide marketing experience or in the literature, not seen in clinical trials, are considered rare and are italicized.)
Body as a Whole: Malaise, swelling of lips and tongue, photosensitivity, anaphylaxis, anaphylactoid reactions.
Cardiovascular: Hypertension, congestive heart failure.
Digestive: Vomiting, jaundice, melena, esophageal lesions, aphthous stomatitis, dry mouth and mucous membranes, bloody diarrhea, hepatitis, hepatic necrosis, cirrhosis, hepatorenal syndrome, appetite change, pancreatitis with or without concomitant hepatitis, colitis.
Hemic and Lymphatic: Hemoglobin decrease, leukopenia, thrombocytopenia, eosinophilia, hemolytic anemia, aplastic anemia, agranulocytosis, purpura, allergic purpura.
Metabolic and Nutritional Disorders: Azotemia.
Nervous System: Insomnia, drowsiness, depression, diplopia, anxiety, irritability, aseptic meningitis, convulsions.
Respiratory: Epistaxis, asthma, laryngeal edema.
Skin and Appendages: Alopecia, urticaria, eczema, dermatitis, bullous eruption, erythema multiforme major, angioedema, Stevens-Johnson syndrome.
Special Senses: Blurred vision, taste disorder, reversible and irreversible hearing loss, scotoma.
Urogenital: Nephrotic syndrome, proteinuria, oliguria, interstitial nephritis, papillary necrosis, acute renal failure.
Incidence Less Than 1% - Causal Relationship Unknown:
(The following reactions have been reported in patients taking diclofenac under circumstances that do not permit a clear attribution of the reaction to diclofenac. These reactions are being included as alerting information to physicians. Adverse reactions reported only in worldwide marketing experience or in the literature, not seen in clinical trials, are considered rare and are italicized).
Body as a Whole: Chest pain.
Cardiovascular: Palpitations, flushing, tachycardia, premature ventricular contractions, myocardial infarction, hypotension.
Digestive: Intestinal perforation.
Hemic and Lymphatic: Bruising.
Metabolic and Nutritional Disorders: Hypoglycemia, weight loss.
Nervous System: Parasthesia, memory disturbance, nightmares, tremor, tic, abnormal coordination, disorientation, psychotic reaction.
Respiratory: Dyspnea, hyperventilation, edema of pharynx.
Skin and Appendages: Excess perspiration, exfoliative dermatitis.
Special Senses: Vitreous floaters, night blindness, amblyopia.
Urogenital: Urinary frequency, nocturia, hematuria, impotence, vaginal bleeding.
OVERDOSAGE
Worldwide reports of overdosage with diclofenac cover 68 cases. In approximately one-half of these reports of overdosage, concomitant medications were also taken. The highest dose of diclofenac was 5 g in a 17-year-old male who suffered loss of consciousness, increased intracranial pressure, aspiration pneumonitis, and died 2 days after overdose. The next highest doses of diclofenac were 4 g and 3.75 g. The 24-year-old female who took 4 g and the 28-and 42-year-old females, each of whom took 3.75 g, did not develop any clinically significant signs or symptoms. However, there was a report of a 17-year-old female who experienced vomiting and drowsiness after an overdose of 2.37 g of diclofenac.
Animal LD50 values show a wide range of susceptibilities to acute overdosage, with primates being more resistant to acute toxicity than rodents (LD50 in mg/kg-rats, 55; dogs, 500; monkeys, 3200).
In case of acute overdosage, it is recommended that the stomach be emptied by vomiting or lavage. Forced diuresis may theoretically be beneficial because the drug is excreted in the urine. The effect of dialysis or hemoperfusion in the elimination of diclofenac (99% protein-bound: see CLINICAL PHARMACOLOGY) remains unproven. In addition to supportive measures, the use of oral activated charcoal may help to reduce the absorption of diclofenac.
DOSAGE AND ADMINISTRATION
Diclofenac may be administered as 25 mg, 50 mg, and 75 mg diclofenac sodium delayed-release tablets. The dosage of diclofenac sodium should be individualized to the lowest effective dose to minimize adverse effects (see INDIVIDUALIZATION OF DOSAGE).
Osteoarthritis:
The recommended dosage is 100 to 150 mg/day in divided doses, 50 mg b.i.d. or t.i.d. or 75 mg b.i.d. Dosages above 200 mg/day have not been studied in patients with osteoarthritis.
HOW SUPPLIED
Diclofenac sodium delayed-release tablets 50 mg - light brown, round, normal convex (imprinted G-DS-50 in black)
Bottles of 60 NDC-57315-015-01
Bottles of 100 NDC-57315-015-05
Bottles of 1000 NDC-57315-015-02
Unit Dose (blister pack)
Box of 100 (strips of 10) NDC-57315-015-04
Diclofenac sodium delayed-release tablets 75 mg – pale pink, round, normal convex (imprinted G-DS-75 in black)
Bottles of 60 NDC-57315-016-01
Bottles of 100 NDC-57315-016-05
Bottles of 1000 NDC-57315-016-02
Unit Dose (blister pack)
Box of 100 (strips of 10) NDC-57315-016-04
Do not store above 30°C (86°F). Protect from moisture. Dispense in a tight, light-resistant container as defined in USP.
Rx only
Manufactured By: ALPHAPHARM PTY LTD
Cnr. Garnet and Antimony Sts.,
Carole Park QLD 4300
Australia
517/3
Call 1-800-661 3429 Revised November 1998
| DICLOFENAC SODIUM DELAYED-RELEASE
diclofenac sodium tablet, delayed release |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| DICLOFENAC SODIUM DELAYED-RELEASE
diclofenac sodium tablet, delayed release |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Labeler - ALPHAPHARM PTY LTD (754819436) |