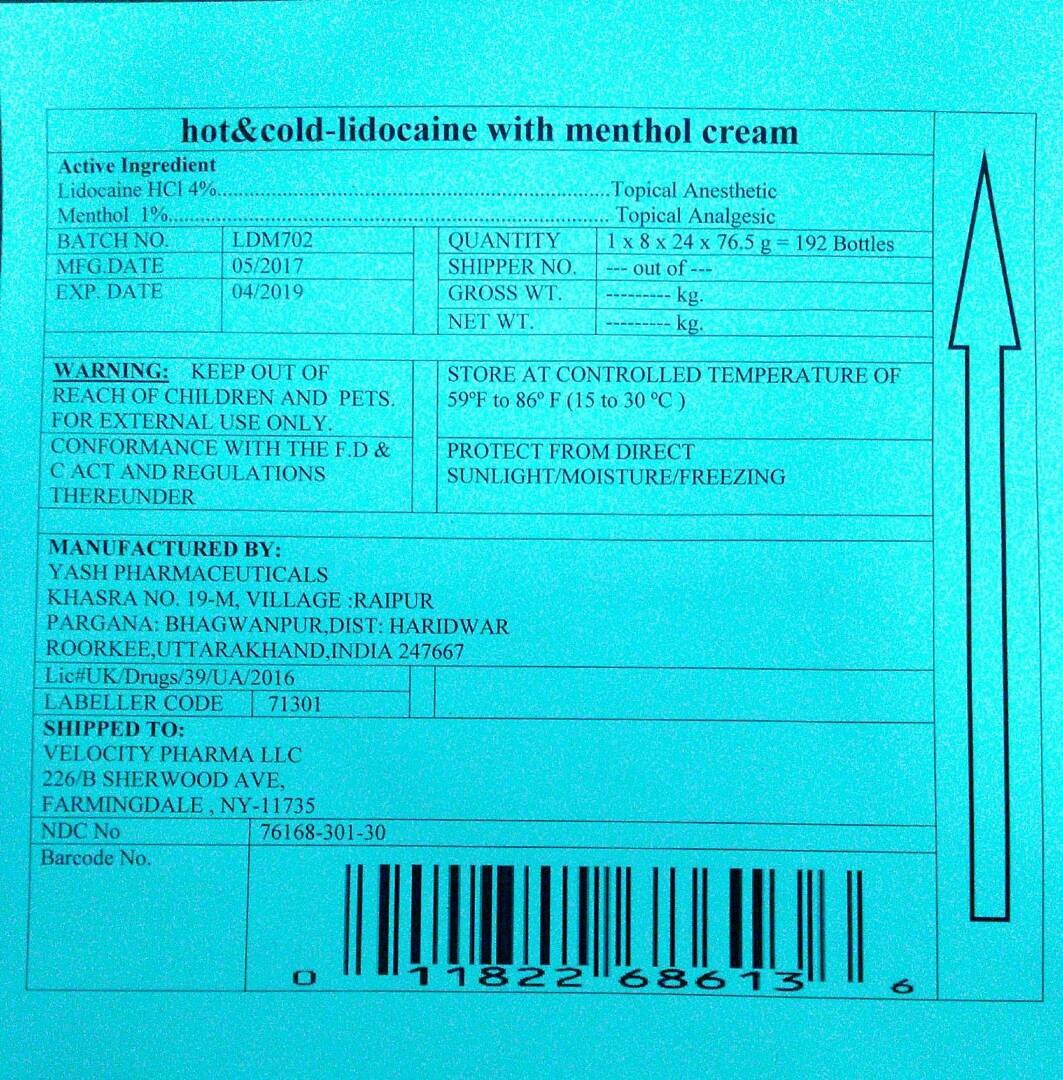
Drug Facts
HOT AND COLD CREAM with LIDOCAINE-RA
PAIN RELIEVING CREAM
Drug Facts
Active Ingredients
Lidocaine HCl 4%
Menthol 1%
Purposes
Lidocaine- Topical anesthetic,
Menthol- Topical Analgesic
WARNINGS
For external use only
DO NOT USE
- on large areas of the body or on cut, irritated or swollen skin
- on puncture wounds
- for more than one week without consulting a doctor
WHEN USING SECTION
- Use only as directed. Read and follow all directions and warnings on this label
- rare cases of serious burns have been reported with products of this type
- do not bandage tightly or apply local heat (such as heating pads) to the area of use or use with a medicated patch
- avoid contact with eyes and mucous membranes
- a transient burning sensation may occur upon application but generally disappears in several days
ask a health professional before use.
Keep out of reach of children and pets. If swallowed, get medical help or contact a Poison Control Center right away.
adults and children over 12 years:
- apply a thin layer to affected area every 6 to 8 hours, not to exceed 3 applications in a 24 hour period
- AFTER APPLYING, WASH HANDS WITH SOAP AND WATER
children 12 years or younger: ask a doctor
Butylated hydroxyl toluene,cetostearyl alcohol,cetomacrogol 1000,cetyl alcohol,disodium EDTA,disodium hydrogen phosphate,light liquid paraffin,propylene glycol,sorbic acid,transquitol P, white petroleum jelly
Keep Carton As It Contains Important Information
Close cap tightly between uses.