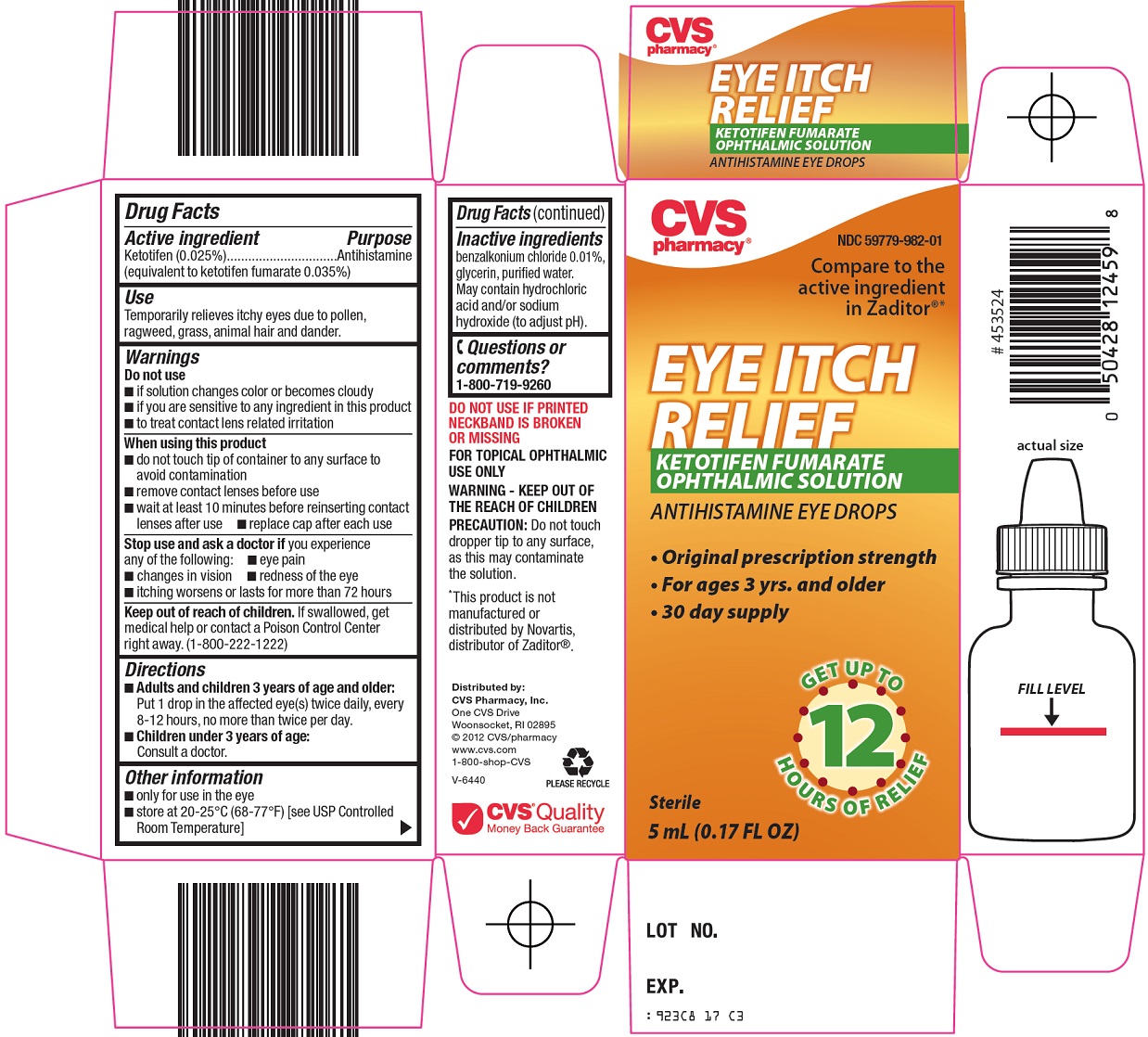
EYE ITCH RELIEF- ketotifen fumarate solution
CVS Pharmacy
----------
CVS Pharmacy, Inc. Eye Itch Relief Drug Facts
Warnings
Do not use
- •
- if solution changes color or becomes cloudy
- •
- if you are sensitive to any ingredient in this product
- •
- to treat contact lens related irritation
When using this product
- •
- do not touch tip of container to any surface to avoid contamination
- •
- remove contact lenses before use
- •
- wait at least 10 minutes before reinserting contact lenses after use
- •
- replace cap after each use
Directions
- •
- Adults and children 3 years of age and older: Put 1 drop in the affected eye(s) twice daily, every 8-12 hours, no more than twice per day.
- •
- Children under 3 years of age:
- Consult a doctor.
Other information
- •
- only for use in the eye
- •
- store at 20-25°C (68-77°F) [see USP Controlled Room Temperature]
| EYE ITCH RELIEF
ketotifen fumarate solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - CVS Pharmacy (062312574) |
Revised: 11/2017
Document Id: 00aade5e-a446-44e9-bfb7-4e5359855745
Set id: 1accbb46-1377-4043-ab6c-58f04439bc95
Version: 3
Effective Time: 20171122
CVS Pharmacy