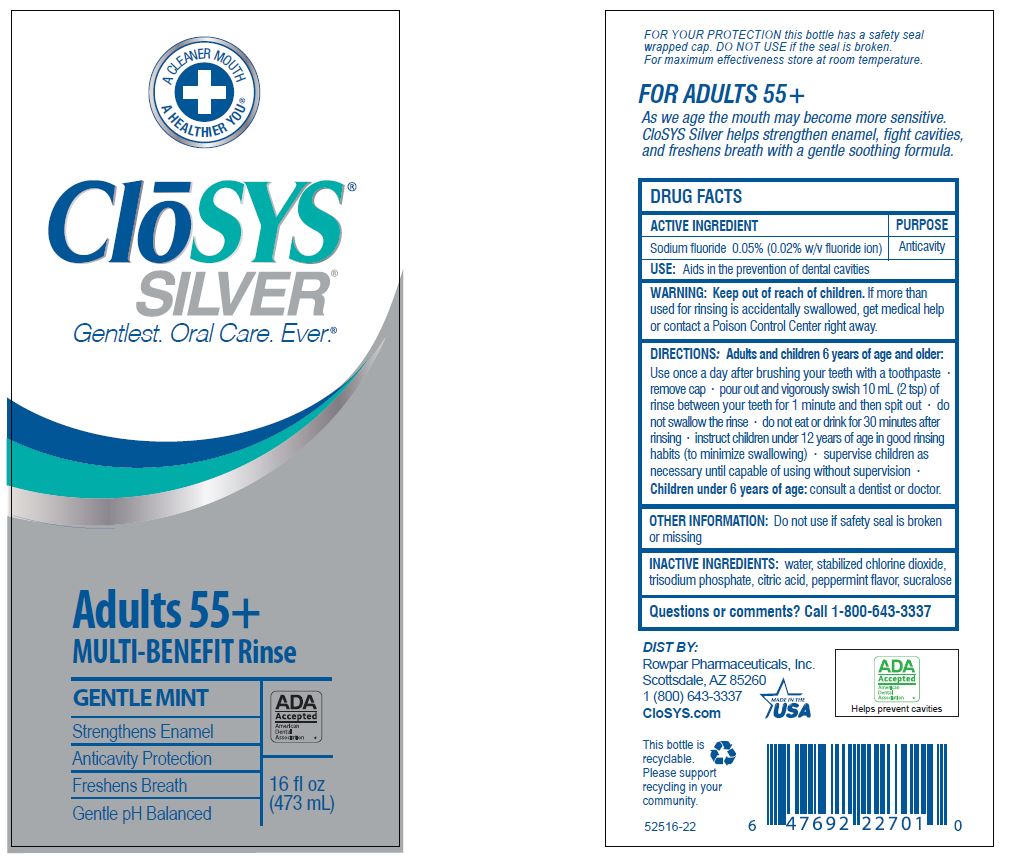
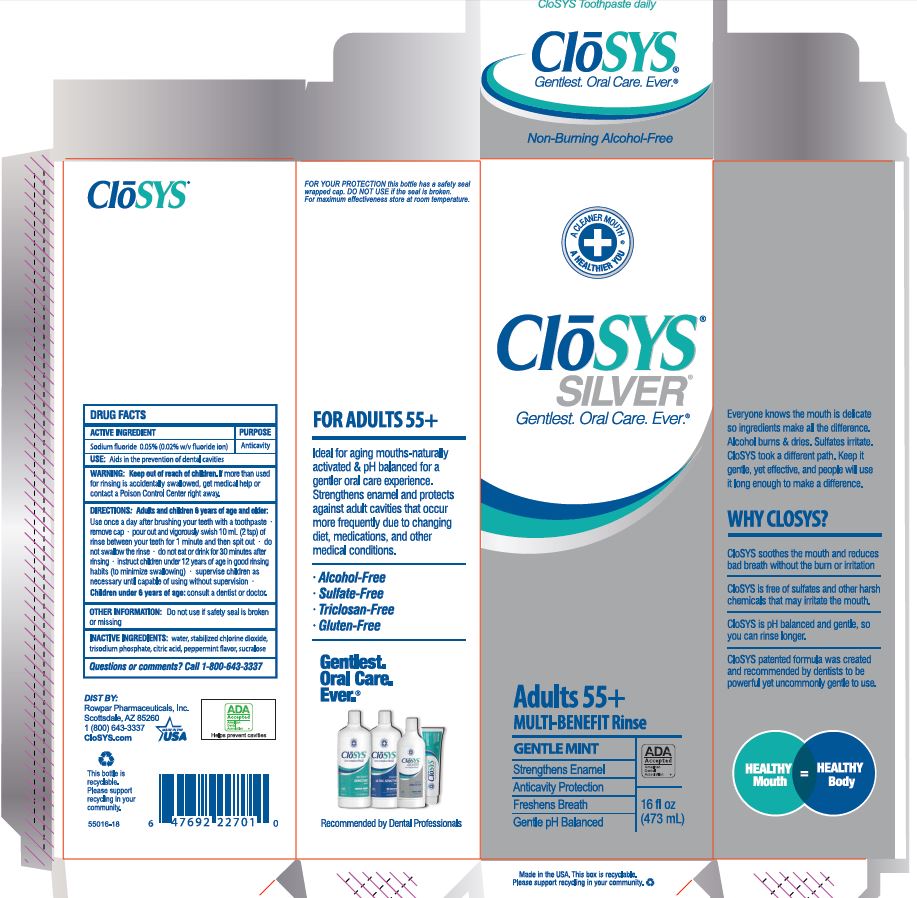
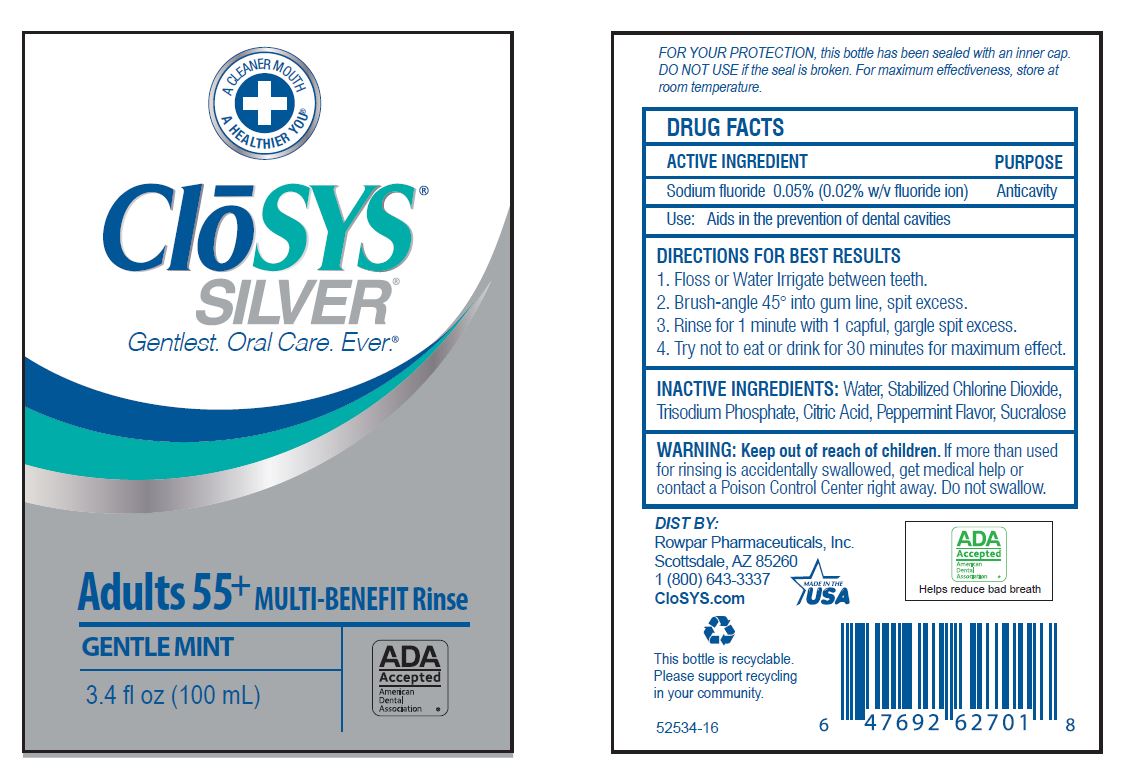
Keep out of reach of children. If more than used for rinsing is accidentally swallowed, get medical help or contact a Poison Control Center right away.
DIRECTIONS: Adults and children 6 years of age and older:
Use once a day after brushing your teeth with a toothpaste · remove cap · pour out and vigorously swish 10 ml (2 tsp) of rinse between your teeth for 1 minute and then spit out · do not swallow the rinse · do not eat or drink for 30 minutes after rinsing • instruct children under 12 years of age in good rinsing habits (to minimize swallowing) · supervise children as necessary until capable of using without supervision ·
Children under 6 years of age: consult a dentist or doctor.